Several thaumatin-like proteins bind to beta-1,3-glucans
- PMID: 9847118
- PMCID: PMC34760
- DOI: 10.1104/pp.118.4.1431
Several thaumatin-like proteins bind to beta-1,3-glucans
Abstract
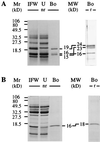
Pathogenesis-related proteins from intercellular fluid washings of stressed barley (Hordeum vulgare L.) leaves were analyzed to determine their binding to various water-insoluble polysaccharides. Three proteins (19, 16, and 15 kD) bound specifically to several water-insoluble beta-1,3-glucans. Binding of the barley proteins to pachyman occurred quickly at 22 degreesC at pH 5.0, even in the presence of 0.5 M NaCl, 0.2 M urea, and 1% (v/v) Triton X-100. Bound barley proteins were released by acidic treatments or by boiling in sodium dodecyl sulfate. Acid-released barley proteins could bind again specifically and singly to pachyman. Water-soluble laminarin and carboxymethyl-pachyman competed for the binding of the barley proteins to pachyman. The N-terminal sequence of the 19-kD barley beta-1,3-glucan-binding protein showed near identity to the barley seed protein BP-R and high homology to other thaumatin-like (TL) permatins. The 16-kD barley protein was also homologous to TL proteins, whereas the 15-kD barley protein N-terminal sequence was identical to the pathogenesis-related Hv-1 TL protein. Antifungal barley protein BP-R and corn (Zea mays) zeamatin were isolated by binding to pachyman. Two extracellular proteins from stressed pea (Pisum sativum L.) also bound to pachyman and were homologous to TL proteins.
Figures



References
-
- Abad LR, D'Urzo MP, Lin D, Narasimhan ML, Renveni M, Zhu JK, Niu X, Singh NK, Hasegawa PM, Bressan RA. Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 1996;118:11–23.
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local aligment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Audy P, Grenier J, Asselin A. Lysozyme activity in animal extracts after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Comp Biochem Physiol. 1989;92B:523–527. - PubMed
-
- Bol JF, Linthorst HJM, Cornelissen BJC. Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol. 1990;28:113–138.
-
- Bussey H. K1 killer toxin, a pore-forming protein from yeast. Mol Microbiol. 1991;5:2339–2343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

