Identification of a domain containing B-cell epitopes in hepatitis C virus E2 glycoprotein by using mouse monoclonal antibodies
- PMID: 9847301
- PMCID: PMC103802
- DOI: 10.1128/JVI.73.1.11-18.1999
Identification of a domain containing B-cell epitopes in hepatitis C virus E2 glycoprotein by using mouse monoclonal antibodies
Abstract
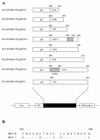
Evidence from clinical and experimental studies of human and chimpanzees suggests that hepatitis C virus (HCV) envelope glycoprotein E2 is a key antigen for developing a vaccine against HCV infection. To identify B-cell epitopes in HCV E2, six murine monoclonal antibodies (MAbs), CET-1 to -6, specific for HCV E2 protein were generated by using recombinant proteins containing E2t (a C-terminally truncated domain of HCV E2 [amino acids 386 to 693] fused to human growth hormone and glycoprotein D). We tested whether HCV-infected sera were able to inhibit the binding of CET MAbs to the former fusion protein. Inhibitory activity was observed in most sera tested, which indicated that CET-1 to -6 were similar to anti-E2 antibodies in human sera with respect to the epitope specificity. The spacial relationship of epitopes on E2 recognized by CET MAbs was determined by surface plasmon resonance analysis and competitive enzyme-linked immunosorbent assay. The data indicated that three overlapping epitopes were recognized by CET-1 to -6. For mapping the epitopes recognized by CET MAbs, we analyzed the reactivities of CET MAbs to six truncated forms and two chimeric forms of recombinant E2 proteins. The data suggest that the epitopes recognized by CET-1 to -6 are located in a small domain of E2 spanning amino acid residues 528 to 546.
Figures


Similar articles
-
Generation and Characterization of Monoclonal Antibodies against a Cyclic Variant of Hepatitis C Virus E2 Epitope 412-422.J Virol. 2016 Jan 27;90(7):3745-59. doi: 10.1128/JVI.02397-15. J Virol. 2016. PMID: 26819303 Free PMC article.
-
Combination of neutralizing monoclonal antibodies against Hepatitis C virus E2 protein effectively blocks virus infection.Virus Res. 2016 Sep 15;224:46-57. doi: 10.1016/j.virusres.2016.08.010. Epub 2016 Aug 26. Virus Res. 2016. PMID: 27574733
-
Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step.J Virol. 2011 Jul;85(14):7005-19. doi: 10.1128/JVI.00586-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543495 Free PMC article.
-
Structural and antigenic definition of hepatitis C virus E2 glycoprotein epitopes targeted by monoclonal antibodies.Clin Dev Immunol. 2013;2013:450963. doi: 10.1155/2013/450963. Epub 2013 Jul 9. Clin Dev Immunol. 2013. PMID: 23935648 Free PMC article. Review.
-
HCV E2 core structures and mAbs: something is still missing.Drug Discov Today. 2014 Dec;19(12):1964-70. doi: 10.1016/j.drudis.2014.08.011. Epub 2014 Aug 27. Drug Discov Today. 2014. PMID: 25172800 Free PMC article. Review.
Cited by
-
Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies.J Virol. 2000 Sep;74(17):8011-7. doi: 10.1128/jvi.74.17.8011-8017.2000. J Virol. 2000. PMID: 10933710 Free PMC article.
-
Induction of Genotype Cross-Reactive, Hepatitis C Virus-Specific, Cell-Mediated Immunity in DNA-Vaccinated Mice.J Virol. 2018 Mar 28;92(8):e02133-17. doi: 10.1128/JVI.02133-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29437963 Free PMC article.
-
Mapping B-cell epitopes of hepatitis C virus E2 glycoprotein using human monoclonal antibodies from phage display libraries.J Virol. 2001 Oct;75(20):9986-90. doi: 10.1128/JVI.75.20.9986-9990.2001. J Virol. 2001. PMID: 11559832 Free PMC article.
-
DNA immunization with fusion genes encoding different regions of hepatitis C virus E2 fused to the gene for hepatitis B surface antigen elicits immune responses to both HCV and HBV.World J Gastroenterol. 2002 Jun;8(3):505-10. doi: 10.3748/wjg.v8.i3.505. World J Gastroenterol. 2002. PMID: 12046080 Free PMC article.
-
Vaccination with dendritic cells pulsed with hepatitis C pseudo particles induces specific immune responses in mice.World J Gastroenterol. 2012 Feb 28;18(8):785-93. doi: 10.3748/wjg.v18.i8.785. World J Gastroenterol. 2012. PMID: 22371638 Free PMC article.
References
-
- Alter H J. The chronic consequences of non-A, non-B hepatitis. In: Seeff L B, Lewis J H, editors. Current perspectives in hepatology. New York, N.Y: Plenum; 1989. pp. 83–97.
-
- Chan S-W, Bye J M, Jackson P, Allain J-P. Human recombinant antibodies specific for hepatitis C virus core and envelope E2 peptides from an immune phage display library. J Gen Virol. 1996;77:2531–2539. - PubMed
-
- Choo Q-L, Kuo G, Ralston R, Weiner A, Chien D, Nest G V, Han J, Berger K, Thudium K, Kuo C, Kansopon J, Mcfarland J, Tabrizi A, Ching K, Moss B, Cummins L B, Houghton M, Muchmore E. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. - PMC - PubMed
-
- Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

