Transactivation of a ribosomal gene by simian virus 40 large-T antigen requires at least three activities of the protein
- PMID: 9847324
- PMCID: PMC103825
- DOI: 10.1128/JVI.73.1.214-224.1999
Transactivation of a ribosomal gene by simian virus 40 large-T antigen requires at least three activities of the protein
Abstract
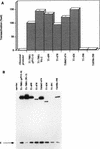
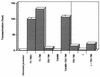
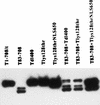
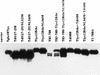
Simian virus 40 large-T antigen transactivates the ribosomal genes which are transcribed by RNA polymerase (pol I), as well as genes that are dependent on either pol II or pol III. This report identifies regions and activities of T antigen that are required to transactivate a pol I-dependent rat ribosomal gene promoter. By using the rat ribosomal gene (rDNA) promoter linked to a chloramphenicol acetyltransferase gene, we show that at least three separable T-antigen regions are necessary to achieve wild-type levels of transactivation of rDNA in transiently transfected monkey cells. One activity depends on the region of T antigen shared with small-t antigen (T/t common region). A second activity maps to amino acids 109 to 626 and is highly sensitive to mutational inactivation. Complementation analyses suggest that at least one activity in this region is independent of and must be in cis with the activity within the T/t common region. In addition, a functional nuclear localization signal is required for maximal T-antigen-mediated transactivation of rat rDNA. The three activities work in concert to override cellular species-specific controls and transactivate the rat ribosomal gene promoter. Finally, we provide evidence that although the tumor suppressor protein Rb has been shown to repress a pol I-dependent promoter, transactivation of the rat rDNA promoter does not depend on T antigen's ability to bind the tumor suppressor product Rb.
Figures






Similar articles
-
Mapping the transcriptional transactivation function of simian virus 40 large T antigen.J Virol. 1991 Jun;65(6):2778-90. doi: 10.1128/JVI.65.6.2778-2790.1991. J Virol. 1991. PMID: 1851853 Free PMC article.
-
Transcriptional activation of the human c-myc gene by simian virus 40 large T antigen without binding to p53 and RB proteins in the transient expression system.Biochem Biophys Res Commun. 1997 Jun 9;235(1):153-7. doi: 10.1006/bbrc.1997.6761. Biochem Biophys Res Commun. 1997. PMID: 9196053
-
Adding an Rb-binding site to an N-terminally truncated simian virus 40 T antigen restores growth to high cell density, and the T common region in trans provides anchorage-independent growth and rapid growth in low serum concentrations.J Virol. 1997 Mar;71(3):1888-96. doi: 10.1128/JVI.71.3.1888-1896.1997. J Virol. 1997. PMID: 9032319 Free PMC article.
-
Molecular chaperone function of the SV40 large T antigen.Dev Biol Stand. 1998;94:313-9. Dev Biol Stand. 1998. PMID: 9776252 Review.
-
SV40 large T antigen functions in DNA replication and transformation.Adv Virus Res. 2000;55:75-134. doi: 10.1016/s0065-3527(00)55002-7. Adv Virus Res. 2000. PMID: 11050941 Review. No abstract available.
Cited by
-
T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis.Microbiol Mol Biol Rev. 2002 Jun;66(2):179-202. doi: 10.1128/MMBR.66.2.179-202.2002. Microbiol Mol Biol Rev. 2002. PMID: 12040123 Free PMC article. Review.
-
The human papillomavirus type 8 E2 tethering protein targets the ribosomal DNA loci of host mitotic chromosomes.J Virol. 2009 Jan;83(2):640-50. doi: 10.1128/JVI.01936-08. Epub 2008 Nov 12. J Virol. 2009. PMID: 19004936 Free PMC article.
-
Single-Cell Transcriptomics Reveals a Heterogeneous Cellular Response to BK Virus Infection.J Virol. 2021 Feb 24;95(6):e02237-20. doi: 10.1128/JVI.02237-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361432 Free PMC article.
-
Regions and activities of simian virus 40 T antigen that cooperate with an activated ras oncogene in transforming primary rat embryo fibroblasts.J Virol. 2002 Apr;76(7):3145-57. doi: 10.1128/jvi.76.7.3145-3157.2002. J Virol. 2002. PMID: 11884539 Free PMC article.
-
Simian virus 40 large T antigen and two independent T-antigen segments sensitize cells to apoptosis following genotoxic damage.J Virol. 2002 Aug;76(16):8420-32. doi: 10.1128/jvi.76.16.8420-8432.2002. J Virol. 2002. PMID: 12134045 Free PMC article.
References
-
- Beckmann H, Chen J-L, O’Brien T, Tjian R. Coactivator and promoter-selective properties of RNA polymerase I TAFs. Science. 1995;270:1506–1509. - PubMed
-
- Bell S P, Jantzen H-M, Tjian R. Assembly of alternative multiprotein complexes directs rRNA promoter selectivity. Genes Dev. 1990;4:943–954. - PubMed
-
- Bell S P, Learned R M, Jantzen H-M, Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988;241:1192–1197. - PubMed
-
- Berger, L. C., D. B. Smith, I. Davidson, J.-J. Hwang, E. Fanning, and A. G. Wildeman. Interaction between T antigen and TEA domain of the factor TEF-1 derepresses simian virus 40 late promoter I vitro: identification of T-antigen domains important to transcription control. J. Virol. 70:1203–1212. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

