Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G
- PMID: 9847327
- PMCID: PMC103828
- DOI: 10.1128/JVI.73.1.242-250.1999
Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G
Abstract
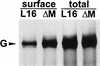
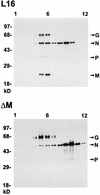
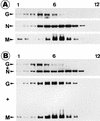
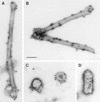
To elucidate the functions of rhabdovirus matrix (M) protein, we determined the localization of M in rabies virus (RV) and analyzed the properties of an M-deficient RV mutant. We provide evidence that M completely covers the ribonucleoprotein (RNP) coil and keeps it in a condensed form. As determined by cosedimentation experiments, not only the M-RNP complex but also M alone was found to interact specifically with the glycoprotein G. In contrast, an interaction of G with the nucleoprotein N or M-less RNP was not observed. In the absence of M, infectious particles were mainly cell associated and the yield of cell-free infectious virus was reduced by as much as 500,000-fold, demonstrating the crucial role of M in virus budding. Supernatants from cells infected with the M-deficient RV did not contain the typical bullet-shaped rhabdovirus particles but instead contained long, rod-shaped virions, demonstrating severe impairment of the virus formation process. Complementation with M protein expressed from plasmids rescued rhabdovirus formation. These results demonstrate the pivotal role of M protein in condensing and targeting the RNP to the plasma membrane as well as in incorporation of G protein into budding virions.
Figures



References
-
- Cartwright B, Smale C J, Brown F. Dissection of vesicular stomatitis virus into the infective ribonucleoprotein and immunizing components. J Gen Virol. 1970;7:19–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

