Human cytomegalovirus inhibits transcription of the CC chemokine MCP-1 gene
- PMID: 9847345
- PMCID: PMC103846
- DOI: 10.1128/JVI.73.1.404-410.1999
Human cytomegalovirus inhibits transcription of the CC chemokine MCP-1 gene
Abstract
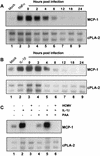
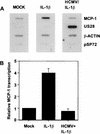
In primary human diploid fibroblasts, infection with an unpurified stock of human cytomegalovirus induced accumulation of the CC chemokine MCP-1 in the cell culture medium. By 24 h postinfection, the level of MCP-1 returned to that in uninfected cultures. When cells were infected with UV-inactivated human cytomegalovirus, the induction of MCP-1 was still observed, but no reduction was seen by 24 h postinfection or later. This effect was the result of a decrease in the level of MCP-1 mRNA present within the infected cell. Infection with purified virus revealed that the induction of MCP-1 was due to an activity found in the medium of infected cells; purified virions did not induce the expression of MCP-1. However, infection with purified virions repressed the level of MCP-1 mRNA below that found in uninfected cells. Additionally, infection with human cytomegalovirus prevented the induction of MCP-1 expression by tumor necrosis factor alpha and interleukin-1beta. The CC chemokine receptor encoded by the human cytomegalovirus US28 open reading frame (ORF) did not appear to play a role in this process, since a mutant virus in which the US28 ORF had been deleted downregulated MCP-1 in the same manner.
Figures






Similar articles
-
Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells.J Exp Med. 1998 Sep 7;188(5):855-66. doi: 10.1084/jem.188.5.855. J Exp Med. 1998. PMID: 9730887 Free PMC article.
-
Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28.J Virol. 2003 Apr;77(8):4489-501. doi: 10.1128/jvi.77.8.4489-4501.2003. J Virol. 2003. PMID: 12663756 Free PMC article.
-
Proinflammatory cytokines induce RANTES and MCP-1 synthesis in human corneal keratocytes but not in corneal epithelial cells. Beta-chemokine synthesis in corneal cells.Invest Ophthalmol Vis Sci. 1996 May;37(6):987-96. Invest Ophthalmol Vis Sci. 1996. PMID: 8631642
-
Human cytomegalovirus directly modulates expression of chemokine CCL2 (MCP-1) during viral replication.J Gen Virol. 2013 Nov;94(Pt 11):2495-2503. doi: 10.1099/vir.0.052878-0. Epub 2013 Aug 12. J Gen Virol. 2013. PMID: 23939977
-
Upregulated expression of interleukin-8, RANTES and chemokine receptors in human astrocytic cells infected with HIV-1.J Neurovirol. 2000 Feb;6(1):75-83. doi: 10.3109/13550280009006384. J Neurovirol. 2000. PMID: 10786999
Cited by
-
Human cytomegalovirus induces a biphasic inflammatory response in primary endothelial cells.J Virol. 2013 Jun;87(11):6530-5. doi: 10.1128/JVI.00265-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536673 Free PMC article.
-
Human cytomegalovirus blocks tumor necrosis factor alpha- and interleukin-1beta-mediated NF-kappaB signaling.J Virol. 2006 Dec;80(23):11686-98. doi: 10.1128/JVI.01168-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005669 Free PMC article.
-
Inhibition of chemokine expression by adenovirus early region three (E3) genes.J Virol. 2002 Aug;76(16):8236-43. doi: 10.1128/jvi.76.16.8236-8243.2002. J Virol. 2002. PMID: 12134029 Free PMC article.
-
Human Cytomegalovirus MicroRNAs miR-US5-1 and miR-UL112-3p Block Proinflammatory Cytokine Production in Response to NF-κB-Activating Factors through Direct Downregulation of IKKα and IKKβ.mBio. 2017 Mar 7;8(2):e00109-17. doi: 10.1128/mBio.00109-17. mBio. 2017. PMID: 28270578 Free PMC article.
-
Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications.J Neurovirol. 2001 Apr;7(2):135-47. doi: 10.1080/13550280152058799. J Neurovirol. 2001. PMID: 11517386
References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1997.
-
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. - PubMed
-
- Bernasconi S, Cinque P, Peri G, Sozzani S, Crociati A, Torri W, Vicenzi E, Vago L, Lazzarin A, Poli G, Mantovani A. Selective elevation of monocyte chemotactic protein-1 in the cerebrospinal fluid of AIDS patients with cytomegalovirus encephalitis. J Infect Dis. 1996;174:1098–1101. - PubMed
-
- Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

