Efficient transduction by an amphotropic retrovirus vector is dependent on high-level expression of the cell surface virus receptor
- PMID: 9847355
- PMCID: PMC103856
- DOI: 10.1128/JVI.73.1.495-500.1999
Efficient transduction by an amphotropic retrovirus vector is dependent on high-level expression of the cell surface virus receptor
Abstract
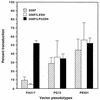
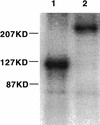
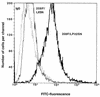
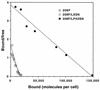
Transduction by murine leukemia virus-based retrovirus vectors is limited in certain cell types, particularly in nondividing cells. But transduction can be inefficient even in cells that divide rapidly. For example, exposure of 208F rat embryo fibroblasts to an excess of an amphotropic retrovirus vector encoding alkaline phosphatase results in a transduction efficiency of only about 10%, even though these cells divide rapidly. Here we show that transduction of 208F cells is limited by cell surface retrovirus receptor levels; overexpression of the amphotropic retrovirus receptor Pit2 markedly improved the transduction efficiency to 50%. To characterize receptor levels and binding affinity, we synthesized a fusion protein that joins the amino terminus of the amphotropic envelope protein to the Fc region of a human immunoglobulin G1 molecule for use in binding assays. In comparison to the parental cell line, the modified cell line showed an order of magnitude increase in binding sites of from 18,000 to 150,000 per cell. Thus, efficient transduction by an amphotropic retrovirus vector requires high-level expression of the retrovirus receptor Pit2. These results provide the rationale for further examination of the role of receptor levels in inefficient transduction, especially with regard to target cells for gene therapy, where a high transduction rate is often crucial.
Figures

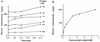
References
-
- Agrawal Y P, Agrawal R S, Sinclair A M, Young D, Maruyama M, Levine F, Ho A D. Cell-cycle kinetics in VSV-G pseudotyped retrovirus-mediated gene transfer in blood-derived CD34+ cells. Exp Hematol. 1996;24:738–747. - PubMed
-
- Badger C C, Krohn K A, Bernstein I D. In vitro measurement of avidity of radioiodinated antibodies. Nucl Med Biol. 1987;6:605–610. - PubMed
-
- Bauer T R, Jr, Miller A D, Hickstein D D. Improved transfer of the leucocyte integrin CD18 subunit into hematopoietic cell lines by using retroviral vectors having a gibbon ape leukemia virus envelope. Blood. 1995;86:2379–2387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

