Sendai virus infection induces apoptosis through activation of caspase-8 (FLICE) and caspase-3 (CPP32)
- PMID: 9847376
- PMCID: PMC103877
- DOI: 10.1128/JVI.73.1.702-708.1999
Sendai virus infection induces apoptosis through activation of caspase-8 (FLICE) and caspase-3 (CPP32)
Abstract
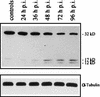
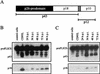
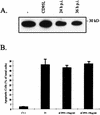
Sendai virus (SV) infection and replication lead to a strong cytopathic effect with subsequent death of host cells. We now show that SV infection triggers an apoptotic program in target cells. Incubation of infected cells with the peptide inhibitor z-VAD-fmk abrogated SV-induced apoptosis, indicating that proteases of the caspase family were involved. Moreover, proteolytic activation of two distinct caspases, CPP32/caspase-3 and, as shown for the first time in virus-infected cells, FLICE/caspase-8, could be detected. So far, activation of FLICE/caspase-8 has been described in apoptosis triggered by death receptors, including CD95 and tumor necrosis factor (TNF)-R1. In contrast, we could show that SV-induced apoptosis did not require TNF or CD95 ligand. We further found that apoptosis of infected cells did not influence the maturation and budding of SV progeny. In conclusion, SV-induced cell injury is mediated by CD95- and TNF-R1-independent activation of caspases, leading to the death of host cells without impairment of the viral life cycle.
Figures



References
-
- Banki K, Hutter E, Gonchoroff N J, Perl A. Molecular ordering in HIV-induced apoptosis—oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J Biol Chem. 1998;273:11944–11953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

