Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro
- PMID: 9847378
- PMCID: PMC103879
- DOI: 10.1128/JVI.73.1.718-727.1999
Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro
Abstract
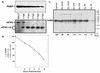
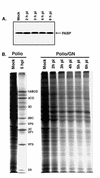
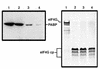
Many enteroviruses, members of the family Picornaviridae, cause a rapid and drastic inhibition of host cell protein synthesis during infection, a process referred to as host cell shutoff. Poliovirus, one of the best-studied enteroviruses, causes marked inhibition of host cell translation while preferentially allowing translation of its own genomic mRNA. An abundance of experimental evidence has accumulated to indicate that cleavage of an essential translation initiation factor, eIF4G, during infection is responsible at least in part for this shutoff. However, evidence from inhibitors of viral replication suggests that an additional event is necessary for the complete translational shutoff observed during productive infection. This report examines the effect of poliovirus infection on a recently characterized 3' end translational stimulatory protein, poly(A)-binding protein (PABP). PABP is involved in stimulating translation initiation in lower eukaryotes by its interaction with the poly(A) tail on mRNAs and has been proposed to facilitate 5'-end-3'-end interactions in the context of the closed-loop translational model. Here, we show that PABP is specifically degraded during poliovirus infection and that it is cleaved in vitro by both poliovirus 2A and 3C proteases and coxsackievirus B3 2A protease. Further, PABP cleavage by 2A protease is accompanied by concurrent loss of translational activity in an in vitro-translation assay. Similar loss of translational activity also occurs simultaneously with partial 3C protease-mediated cleavage of PABP in translation assays. Further, PABP is not degraded during infections in the presence of guanidine-HCl, which blocks the complete development of host translation shutoff. These results provide preliminary evidence that cleavage of PABP may contribute to inhibition of host translation in infected HeLa cells, and they are consistent with the hypothesis that PABP plays a role in facilitating translation initiation in higher eukaryotes.
Figures
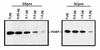
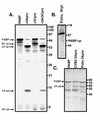


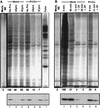
References
-
- Bag J, Wu J. Translational control of poly(A)-binding protein expression. Eur J Biochem. 1996;237:143–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

