Truncated particles produced in fish surviving infectious hematopoietic necrosis virus infection: mediators of persistence?
- PMID: 9847400
- PMCID: PMC103901
- DOI: 10.1128/JVI.73.1.843-849.1999
Truncated particles produced in fish surviving infectious hematopoietic necrosis virus infection: mediators of persistence?
Abstract
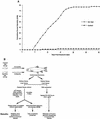
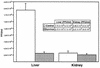
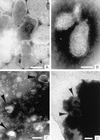
Infectious hematopoietic necrosis virus (IHNV) is a rhabdovirus that produces an acute, lethal infection in rainbow trout (Oncorhynchus mykiss). Fish that survive infection cease to produce detectable infectious virus at approximately 46 days after infection, yet there is evidence that survivor fish continue to harbor virus particles (B. S. Drolet, P. P. Chiou, J. Heidel, and J. C. Leong, J. Virol. 69:2140-2147, 1995). In an effort to determine the biological function of these particles, the kidneys and livers from IHNV survivors were harvested and divided into samples for nested reverse transcriptase PCR analysis and explant culture. Sequences for the IHNV nucleoprotein and polymerase genes were detected in 50 and 89%, respectively, of the organs from survivor fish. When explant tissue cultures were infected with purified standard IHNV, the liver tissues from survivor fish produced up to 10-fold less virus than naive control fish liver tissues. In addition, immunosorbent electron microscopy analysis of the supernatant media from the cultured explants of survivor fish revealed truncated particles, whereas the control tissue supernatants contained only standard viral particles. These results suggest that the truncated IHNV particles observed in persistently infected fish are defective interfering particles that may mediate virus persistence.
Figures

Similar articles
-
Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): a review.Vet Res. 2016 Jun 10;47(1):63. doi: 10.1186/s13567-016-0341-1. Vet Res. 2016. PMID: 27287024 Free PMC article. Review.
-
Detection of truncated virus particles in a persistent RNA virus infection in vivo.J Virol. 1995 Apr;69(4):2140-7. doi: 10.1128/JVI.69.4.2140-2147.1995. J Virol. 1995. PMID: 7884861 Free PMC article.
-
Detection and quantification of infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss) by SYBR Green real-time reverse transcriptase-polymerase chain reaction.J Virol Methods. 2008 Jan;147(1):157-66. doi: 10.1016/j.jviromet.2007.08.026. Epub 2007 Oct 22. J Virol Methods. 2008. PMID: 17945355
-
Virulence of infectious hematopoietic necrosis virus and Infectious pancreatic necrosis virus coinfection in rainbow trout ( Oncorhynchus mykiss) and nucleotide sequence analysis of the IHNV glycoprotein gene.Arch Virol. 2003 Aug;148(8):1507-21. doi: 10.1007/s00705-003-0116-7. Arch Virol. 2003. PMID: 12898328
-
Viruses Infecting the European Catfish (Silurus glanis).Viruses. 2021 Sep 18;13(9):1865. doi: 10.3390/v13091865. Viruses. 2021. PMID: 34578446 Free PMC article. Review.
Cited by
-
Inhibition of infectious haematopoietic necrosis virus in cell cultures with peptide-conjugated morpholino oligomers.J Fish Dis. 2005 Jul;28(7):399-410. doi: 10.1111/j.1365-2761.2005.00641.x. J Fish Dis. 2005. PMID: 16083445 Free PMC article.
-
An overview of process intensification and thermo stabilization for upscaling of Peste des petits ruminants vaccines in view of global control and eradication.Virusdisease. 2018 Sep;29(3):285-296. doi: 10.1007/s13337-018-0455-3. Epub 2018 May 17. Virusdisease. 2018. PMID: 30159362 Free PMC article. Review.
-
Infected juvenile salmon can experience increased predation during freshwater migration.R Soc Open Sci. 2021 Mar 24;8(3):201522. doi: 10.1098/rsos.201522. R Soc Open Sci. 2021. PMID: 33959321 Free PMC article.
-
Viruses of lower vertebrates.J Vet Med B Infect Dis Vet Public Health. 2001 Aug;48(6):403-75. doi: 10.1046/j.1439-0450.2001.00473.x. J Vet Med B Infect Dis Vet Public Health. 2001. PMID: 11550762 Free PMC article. Review.
-
Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): a review.Vet Res. 2016 Jun 10;47(1):63. doi: 10.1186/s13567-016-0341-1. Vet Res. 2016. PMID: 27287024 Free PMC article. Review.
References
-
- Barrera J C, Letchworth G J. Persistence of vesicular stomatitis virus New Jersey RNA in convalescent hamsters. Virology. 1996;219:453–464. - PubMed
-
- Barrett A D, Dimmock N J. Defective interfering viruses and infections of animals. Curr Top Microbiol Immunol. 1986;128:55–84. - PubMed
-
- Buckmeier M J, Welsh R M, Dutko F J, Oldstone M B A. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

