Mutational analysis of the Escherichia coli K-12 TolA N-terminal region and characterization of its TolQ-interacting domain by genetic suppression
- PMID: 9851983
- PMCID: PMC107741
- DOI: 10.1128/JB.180.24.6433-6439.1998
Mutational analysis of the Escherichia coli K-12 TolA N-terminal region and characterization of its TolQ-interacting domain by genetic suppression
Abstract
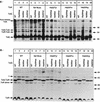
The Tol-Pal proteins of Escherichia coli are involved in maintaining outer membrane integrity. They form two complexes in the cell envelope. Transmembrane domains of TolQ, TolR, and TolA interact in the cytoplasmic membrane, while TolB and Pal form a complex near the outer membrane. The N-terminal transmembrane domain of TolA anchors the protein to the cytoplasmic membrane and interacts with TolQ and TolR. Extensive mutagenesis of the N-terminal part of TolA was carried out to characterize the residues involved in such processes. Mutations affecting the function of TolA resulted in a lack or an alteration in TolA-TolQ or TolR-TolA interactions but did not affect the formation of TolQ-TolR complexes. Our results confirmed the importance of residues serine 18 and histidine 22, which are part of an SHLS motif highly conserved in the TolA and the related TonB proteins from different organisms. Genetic suppression experiments were performed to restore the functional activity of some tolA mutants. The suppressor mutations all affected the first transmembrane helix of TolQ. These results confirmed the essential role of the transmembrane domain of TolA in triggering interactions with TolQ and TolR.
Figures


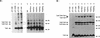
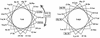
Similar articles
-
Role of TolR N-terminal, central, and C-terminal domains in dimerization and interaction with TolA and tolQ.J Bacteriol. 1999 Aug;181(15):4476-84. doi: 10.1128/JB.181.15.4476-4484.1999. J Bacteriol. 1999. PMID: 10419942 Free PMC article.
-
Protein complex within Escherichia coli inner membrane. TolA N-terminal domain interacts with TolQ and TolR proteins.J Biol Chem. 1995 May 12;270(19):11078-84. doi: 10.1074/jbc.270.19.11078. J Biol Chem. 1995. PMID: 7744737
-
The TolQ-TolR proteins energize TolA and share homologies with the flagellar motor proteins MotA-MotB.Mol Microbiol. 2001 Nov;42(3):795-807. doi: 10.1046/j.1365-2958.2001.02673.x. Mol Microbiol. 2001. PMID: 11722743
-
The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability.FEMS Microbiol Lett. 1999 Aug 15;177(2):191-7. doi: 10.1111/j.1574-6968.1999.tb13731.x. FEMS Microbiol Lett. 1999. PMID: 10474183 Review.
-
Energetics of colicin import revealed by genetic cross-complementation between the Tol and Ton systems.Biochem Soc Trans. 2012 Dec 1;40(6):1480-5. doi: 10.1042/BST20120181. Biochem Soc Trans. 2012. PMID: 23176502 Review.
Cited by
-
Localization of the outer membrane protein OmpA2 in Caulobacter crescentus depends on the position of the gene in the chromosome.J Bacteriol. 2014 Aug;196(15):2889-900. doi: 10.1128/JB.01516-14. Epub 2014 Jun 2. J Bacteriol. 2014. PMID: 24891444 Free PMC article.
-
The multifarious roles of Tol-Pal in Gram-negative bacteria.FEMS Microbiol Rev. 2020 Jul 1;44(4):490-506. doi: 10.1093/femsre/fuaa018. FEMS Microbiol Rev. 2020. PMID: 32472934 Free PMC article. Review.
-
Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity.J Bacteriol. 2002 Feb;184(3):754-9. doi: 10.1128/JB.184.3.754-759.2002. J Bacteriol. 2002. PMID: 11790745 Free PMC article.
-
Recruitment of the TolA Protein to Cell Constriction Sites in Escherichia coli via Three Separate Mechanisms, and a Critical Role for FtsWI Activity in Recruitment of both TolA and TolQ.J Bacteriol. 2022 Jan 18;204(1):e0046421. doi: 10.1128/JB.00464-21. Epub 2021 Nov 8. J Bacteriol. 2022. PMID: 34748387 Free PMC article.
-
Mapping the interactions between Escherichia coli TolQ transmembrane segments.J Biol Chem. 2011 Apr 1;286(13):11756-64. doi: 10.1074/jbc.M110.192773. Epub 2011 Feb 1. J Biol Chem. 2011. PMID: 21285349 Free PMC article.
References
-
- Bénédetti H, Géli V. Colicin transport, channel formation and inhibition. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. Vol. 2. Amsterdam, The Netherlands: Elsevier Science B.V.; 1996. pp. 665–691.
-
- Bouveret E, Derouiche R, Rigal A, Lloubès R, Lazdunski C, Bénédetti H. Peptidoglycan-associated lipoprotein-TolB interaction. A possible key to explaining the formation of contact sites between the inner and outer membranes of Escherichia coli. J Biol Chem. 1995;270:11071–11077. - PubMed
-
- Bouveret E, Rigal A, Lazdunski C, Bénédetti H. The N-terminal domain of colicin E3 interacts with TolB which is involved in the colicin translocation step. Mol Microbiol. 1997;23:909–920. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

