Suppression of an Hsp70 mutant phenotype in Saccharomyces cerevisiae through loss of function of the chromatin component Sin1p/Spt2p
- PMID: 9851990
- PMCID: PMC107749
- DOI: 10.1128/JB.180.24.6484-6492.1998
Suppression of an Hsp70 mutant phenotype in Saccharomyces cerevisiae through loss of function of the chromatin component Sin1p/Spt2p
Abstract
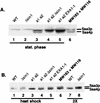
The Ssa subfamily of Hsp70 molecular chaperones in the budding yeast Saccharomyces cerevisiae has four members, encoded by SSA1, SSA2, SSA3, and SSA4. Deletion of the two constitutively expressed genes, SSA1 and SSA2, results in cells which are slow growing and temperature sensitive. In this study, we demonstrate that an extragenic suppressor of the temperature sensitivity of ssa1 ssa2 strains, EXA1-1, is a loss-of-function mutation in SIN1/SPT2, which encodes a nonhistone component of chromatin. Loss of function of Sin1p leads to overexpression of SSA3 in the ssa1 ssa2 mutant background, at a level which is sufficient to mediate suppression. In a strain which is wild type for SSA genes, we detected no effect of Sin1p on Ssa3p expression except under conditions of heat shock. Existing data indicate that expression of SSA3 in the ssa1 ssa2 mutant background as well as in heat-shocked wild-type strains is mediated by the heat shock transcription factor HSF. Our findings suggest that it is HSF-mediated induction of SSA3 which is modulated by Sin1p. The EXA1-1 suppressor mutation thus improves the growth of ssa1 ssa2 strains by selectively increasing HSF-mediated expression of SSA3.
Figures






References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1997.
-
- Bollag D M, Edelstein S J. Protein methods. New York, N.Y: Wildy-Liss, Inc.; 1991.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

