Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon
- PMID: 9852001
- PMCID: PMC107760
- DOI: 10.1128/JB.180.24.6571-6580.1998
Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon
Abstract
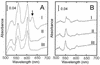
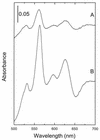
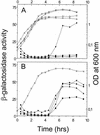
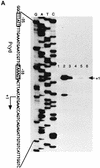
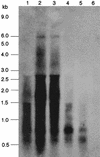
Under aerobic conditions Bacillus subtilis utilizes a branched electron transport chain comprising various cytochromes and terminal oxidases. At present there is evidence for three types of terminal oxidases in B. subtilis: a caa3-, an aa3-, and a bd-type oxidase. We report here the cloning of the structural genes (cydA and cydB) encoding the cytochrome bd complex. Downstream of the structural genes, cydC and cydD are located. These genes encode proteins showing similarity to bacterial ATP-binding cassette (ABC)-type transporters. Analysis of isolated cell membranes showed that inactivation of cydA or deletion of cydABCD resulted in the loss of spectral features associated with cytochrome bd. Gene disruption experiments and complementation analysis showed that the cydC and cydD gene products are required for the expression of a functional cytochrome bd complex. Disruption of the cyd genes had no apparent effect on the growth of cells in broth or defined media. The expression of the cydABCD operon was investigated by Northern blot analysis and by transcriptional and translational cyd-lacZ fusions. Northern blot analysis confirmed that cydABCD is transcribed as a polycistronic message. The operon was found to be expressed maximally under conditions of low oxygen tension.
Figures




References
-
- Cole S T, Brosch R, Parhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas G, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingwort T, Conner R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. - PubMed
-
- Cook G M, Membrillo-Hernandez J, Poole R K. Transcriptional regulation of the cydDC operon, encoding a heterodimeric ABC transporter required for assembly of cytochromes c and bd in Escherichia coli K-12: regulation by oxygen and alternative electron acceptors. J Bacteriol. 1997;179:6525–6530. - PMC - PubMed
-
- Cunningham L, Pitt M, Williams H D. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol Microbiol. 1997;24:579–591. - PubMed
-
- Dahl M K, Meinhof C-G. A series of integrative plasmids for Bacillus subtilis containing unique cloning sites in all three open reading frames for translational lacZ fusions. Gene. 1994;145:151–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

