The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor sigmaS and the stress response in Pseudomonas fluorescens Pf-5
- PMID: 9852008
- PMCID: PMC107767
- DOI: 10.1128/JB.180.24.6635-6641.1998
The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor sigmaS and the stress response in Pseudomonas fluorescens Pf-5
Abstract
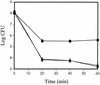
Three global regulators are known to control antibiotic production by Pseudomonas fluorescens. A two-component regulatory system comprised of the sensor kinase GacS (previously called ApdA or LemA) and GacA, a member of the FixJ family of response regulators, is required for antibiotic production. A mutation in rpoS, which encodes the stationary-phase sigma factor sigmaS, differentially affects antibiotic production and reduces the capacity of stationary-phase cells of P. fluorescens to survive exposure to oxidative stress. The gacA gene of P. fluorescens Pf-5 was isolated, and the influence of gacS and gacA on rpoS transcription, sigmaS levels, and oxidative stress response of Pf-5 was determined. We selected a gacA mutant of Pf-5 that contained a single nucleotide substitution within a predicted alpha-helical region, which is highly conserved among the FixJ family of response regulators. At the entrance to stationary phase, sigmaS content in gacS and gacA mutants of Pf-5 was less than 20% of the wild-type level. Transcription of rpoS, assessed with an rpoS-lacZ transcriptional fusion, was positively influenced by GacS and GacA, an effect that was most evident at the transition between exponential growth and stationary phase. Mutations in gacS and gacA compromised the capacity of stationary-phase cells of Pf-5 to survive exposure to oxidative stress. The results of this study provide evidence for the predominant roles of GacS and GacA in the regulatory cascade controlling stress response and antifungal metabolite production in P. fluorescens.
Figures
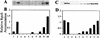
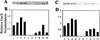

References
-
- Alvarado-Urbina G, Chiarello R, Roberts E, Vilain G, Jurik F, Christensen L, Carmona C, Fang L, Watterson M, Crea R. Rapid automated synthesis via diisopropyl phosporamidite in situ activation. Chemical synthesis and cloning of a calmodulin gene. Biochem Cell Biol. 1986;64:548–555. - PubMed
-
- Black T, Lam S, Gaffney T. Abstract book, 8th International Congress of Molecular Plant-Microbe Interactions, Knoxville, Tenn. 1996. Transcriptional analysis of LemA, GacA, RpoS and GacA-regulated genes in Pseudomonas fluorescens BL915.
-
- Bull, C. T. 1998. Personal communication.
-
- Chou P Y, Fasman G D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–147. - PubMed
-
- Corbell, N. A. Unpublished data.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

