Comparison in vitro of a high- and a low-abundance chemoreceptor of Escherichia coli: similar kinase activation but different methyl-accepting activities
- PMID: 9852019
- PMCID: PMC107778
- DOI: 10.1128/JB.180.24.6713-6718.1998
Comparison in vitro of a high- and a low-abundance chemoreceptor of Escherichia coli: similar kinase activation but different methyl-accepting activities
Abstract
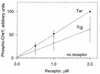
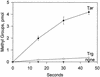
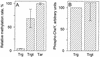
In Escherichia coli, high-abundance chemoreceptors are present in cellular amounts approximately 10-fold greater than low-abundance chemoreceptors. Cells containing only low-abundance receptors exhibit abnormally low tumble frequencies and do not migrate effectively in spatial gradients. These defects reflect an inherent activity difference between the two receptor classes. We used in vitro assays to investigate this difference. The low-abundance receptor Trg mediated an approximately 100-fold activation of the kinase CheA, only twofold less than activation by the high-abundance receptor Tar. In contrast, Trg was less than 1/20 as active as Tar for in vitro methylation. As observed for high-abundance receptors, kinase activation by Trg varied with the extend of modification at methyl-accepting sites; low methylation corresponded to low kinase activation. Thus, in Trg-only cells, low receptor methylation would result in low kinase activation, correspondingly low content of phospho-CheY, and a decreased dynamic range over which attractant binding could modulate kinase activity. These features could account for the low tumble frequency and inefficient taxis exhibited by Trg-only cells. Thus, the crucial functional difference between the receptor classes is likely to be methyl-accepting activity. We investigated the structural basis for this functional difference by introducing onto the carboxy terminus of Trg a CheR-binding pentapeptide, usually found only at the carboxy termini of high-abundance receptors. This addition enhanced the in vitro methyl-accepting activity of Trg 10-fold.
Figures
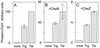

References
-
- Alex L A, Simon M I. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. - PubMed
-
- Ames P, Yu Y A, Parkinson J S. Methylation segments are not required for chemotactic signaling by cytoplasmic fragments of Tsr, the methyl-accepting serine chemoreceptor of Escherichia coli. Mol Microbiol. 1996;19:737–746. - PubMed
-
- Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

