The localization of myosin VI at the golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation
- PMID: 9852149
- PMCID: PMC2132970
- DOI: 10.1083/jcb.143.6.1535
The localization of myosin VI at the golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation
Abstract
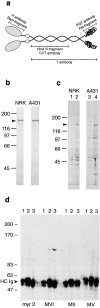
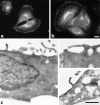
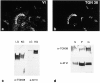
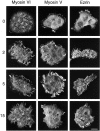
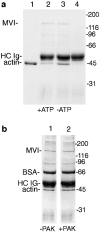
Myosin VI is an unconventional myosin that may play a role in vesicular membrane traffic through actin rich regions of the cytoplasm in eukaryotic cells. In this study we have cloned and sequenced a cDNA encoding a chicken intestinal brush border myosin VI. Polyclonal antisera were raised to bacterially expressed fragments of this myosin VI. The affinity purified antibodies were highly specific for myosin VI by immunoblotting and immunoprecipitation and were used to study the localization of the protein by immunofluorescence and immunoelectron microscopy. It was found that in NRK and A431 cells, myosin VI was associated with both the Golgi complex and the leading, ruffling edge of the cell as well as being present in a cytosolic pool. In A431 cells in which cell surface ruffling was stimulated by EGF, myosin VI was phosphorylated and recruited into the newly formed ruffles along with ezrin and myosin V. In vitro experiments suggested that a p21-activated kinase (PAK) might be the kinase responsible for phosphorylation in the motor domain. These results strongly support a role for myosin VI in membrane traffic on secretory and endocytic pathways.
Figures


References
-
- Allan V. Role of motor proteins in organizing the endoplasmic reticulum and Golgi apparatus. Semin Cell Devel Biol. 1996;7:335–342.
-
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell's waltzerdeafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11:369–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

