Heterotrimeric kinesin II is the microtubule motor protein responsible for pigment dispersion in Xenopus melanophores
- PMID: 9852150
- PMCID: PMC2132968
- DOI: 10.1083/jcb.143.6.1547
Heterotrimeric kinesin II is the microtubule motor protein responsible for pigment dispersion in Xenopus melanophores
Abstract
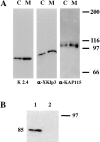
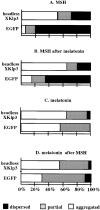
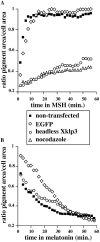
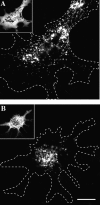
Melanophores move pigment organelles (melanosomes) from the cell center to the periphery and vice-versa. These bidirectional movements require cytoplasmic microtubules and microfilaments and depend on the function of microtubule motors and a myosin. Earlier we found that melanosomes purified from Xenopus melanophores contain the plus end microtubule motor kinesin II, indicating that it may be involved in dispersion (Rogers, S.L., I.S. Tint, P.C. Fanapour, and V.I. Gelfand. 1997. Proc. Natl. Acad. Sci. USA. 94: 3720-3725). Here, we generated a dominant-negative construct encoding green fluorescent protein fused to the stalk-tail region of Xenopus kinesin-like protein 3 (Xklp3), the 95-kD motor subunit of Xenopus kinesin II, and introduced it into melanophores. Overexpression of the fusion protein inhibited pigment dispersion but had no effect on aggregation. To control for the specificity of this effect, we studied the kinesin-dependent movement of lysosomes. Neither dispersion of lysosomes in acidic conditions nor their clustering under alkaline conditions was affected by the mutant Xklp3. Furthermore, microinjection of melanophores with SUK4, a function-blocking kinesin antibody, inhibited dispersion of lysosomes but had no effect on melanosome transport. We conclude that melanosome dispersion is powered by kinesin II and not by conventional kinesin. This paper demonstrates that kinesin II moves membrane-bound organelles.
Figures



References
-
- Cole DG, Scholey JM. Structural variations among the kinesins. Trends Cell Biol. 1995;5:259–262. - PubMed

