Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells
- PMID: 9852152
- PMCID: PMC2132987
- DOI: 10.1083/jcb.143.6.1575
Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells
Abstract
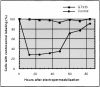
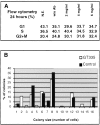
Glutamylation is the major posttranslational modification of neuronal and axonemal tubulin and is restricted predominantly to centrioles in nonneuronal cells (Bobinnec, Y., M. Moudjou, J.P. Fouquet, E. Desbruyères, B. Eddé, and M. Bornens. 1998. Cell Motil. Cytoskel. 39:223-232). To investigate a possible relationship between the exceptional stability of centriole microtubules and the compartmentalization of glutamylated isoforms, we loaded HeLa cells with the monoclonal antibody GT335, which specifically reacts with polyglutamylated tubulin. The total disappearance of the centriole pair was observed after 12 h, as judged both by immunofluorescence labeling with specific antibodies and electron microscopic observation of cells after complete thick serial sectioning. Strikingly, we also observed a scattering of the pericentriolar material (PCM) within the cytoplasm and a parallel disappearance of the centrosome as a defined organelle. However, centriole disappearance was transient, as centrioles and discrete centrosomes ultimately reappeared in the cell population. During the acentriolar period, a large proportion of monopolar half-spindles or of bipolar spindles with abnormal distribution of PCM and NuMA were observed. However, as judged by a quasinormal increase in cell number, these cells likely were not blocked in mitosis. Our results suggest that a posttranslational modification of tubulin is critical for long-term stability of centriolar microtubules. They further demonstrate that in animal cells, centrioles are instrumental in organizing centrosomal components into a structurally stable organelle.
Figures
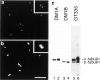
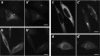
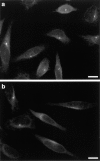

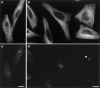






References
-
- Audebert S, Koulakoff A, Berwald-Netter Y, Gros F, Denoulet P, Eddé B. Developmental regulation of polyglutamylated α- and β-tubulin in mouse brain neurons. J Cell Sci. 1994;107:2313–2322. - PubMed
-
- Baron AT, Greenwood TM, Bazinet CW, Salisbury JL. Centrin is a component of the pericentriolar lattice. Biol Cell. 1992;76:383–388. - PubMed
-
- Beatty, R.A. 1967. Parthenogenesis in vertebrates. In Fertilization. C.B. Metz and A. Monroy, editors. Academic Press, New York. 413–440.
-
- Bobinnec Y, Moudjou M, Fouquet JP, Desbruyères E, Eddé B, Bornens M. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil Cytoskel. 1998;39:223–232. - PubMed
-
- Bornens, M. 1992. Structure and function of isolated centrosomes. In The Centrosome. V.I. Kalnins, editor. Academic Press, San Diego. 1–43.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

