Role of polo kinase and Mid1p in determining the site of cell division in fission yeast
- PMID: 9852154
- PMCID: PMC2132972
- DOI: 10.1083/jcb.143.6.1603
Role of polo kinase and Mid1p in determining the site of cell division in fission yeast
Abstract
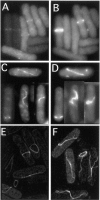
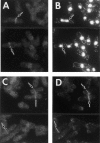
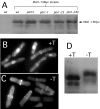
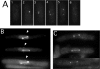
The fission yeast Schizosaccharomyces pombe divides symmetrically using a medial F-actin- based contractile ring to produce equal-sized daughter cells. Mutants defective in two previously described genes, mid1 and pom1, frequently divide asymmetrically. Here we present the identification of three new temperature-sensitive mutants defective in localization of the division plane. All three mutants have mutations in the polo kinase gene, plo1, and show defects very similar to those of mid1 mutants in both the placement and organization of the medial ring. In both cases, ring formation is frequently initiated near the cell poles, indicating that Mid1p and Plo1p function in recruiting medial ring components to the cell center. It has been reported previously that during mitosis Mid1p becomes hyperphosphorylated and relocates from the nucleus to a medial ring. Here we show that Mid1p first forms a diffuse cortical band during spindle formation and then coalesces into a ring before anaphase. Plo1p is required for Mid1p to exit the nucleus and form a ring, and Pom1p is required for proper placement of the Mid1p ring. Upon overexpression of Plo1p, Mid1p exits the nucleus prematurely and displays a reduced mobility on gels similar to that of the hyperphosphorylated form observed previously in mitotic cells. Genetic and two-hybrid analyses suggest that Plo1p and Mid1p act in a common pathway distinct from that involving Pom1p. Plo1p localizes to the spindle pole bodies and spindles of mitotic cells and also to the medial ring at the time of its formation. Taken together, the data indicate that Plo1p plays a role in the positioning of division sites by regulating Mid1p. Given its previously known functions in mitosis and the timing of cytokinesis, Plo1p is thus implicated as a key molecule in the spatial and temporal coordination of cytokinesis with mitosis.
Figures





References
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1995. Current Protocols in Molecular Biology. John Wiley and Sons Ltd., New York.
-
- Bähler J, Wu J-Q, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. . Yeast. 1998;14:943–951. - PubMed
-
- Balasubramanian MK, McCollum D, Gould KL. Cytokinesis in the fission yeast Schizosaccharomyces pombe. . Methods Enzymol. 1997;283:494–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

