AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos
- PMID: 9852156
- PMCID: PMC2132979
- DOI: 10.1083/jcb.143.6.1635
AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos
Abstract
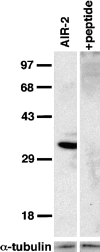
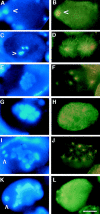
An emerging family of kinases related to the Drosophila Aurora and budding yeast Ipl1 proteins has been implicated in chromosome segregation and mitotic spindle formation in a number of organisms. Unlike other Aurora/Ipl1-related kinases, the Caenorhabditis elegans orthologue, AIR-2, is associated with meiotic and mitotic chromosomes. AIR-2 is initially localized to the chromosomes of the most mature prophase I-arrested oocyte residing next to the spermatheca. This localization is dependent on the presence of sperm in the spermatheca. After fertilization, AIR-2 remains associated with chromosomes during each meiotic division. However, during both meiotic anaphases, AIR-2 is present between the separating chromosomes. AIR-2 also remains associated with both extruded polar bodies. In the embryo, AIR-2 is found on metaphase chromosomes, moves to midbody microtubules at anaphase, and then persists at the cytokinesis remnant. Disruption of AIR-2 expression by RNA- mediated interference produces entire broods of one-cell embryos that have executed multiple cell cycles in the complete absence of cytokinesis. The embryos accumulate large amounts of DNA and microtubule asters. Polar bodies are not extruded, but remain in the embryo where they continue to replicate. The cytokinesis defect appears to be late in the cell cycle because transient cleavage furrows initiate at the proper location, but regress before the division is complete. Additionally, staining with a marker of midbody microtubules revealed that at least some of the components of the midbody are not well localized in the absence of AIR-2 activity. Our results suggest that during each meiotic and mitotic division, AIR-2 may coordinate the congression of metaphase chromosomes with the subsequent events of polar body extrusion and cytokinesis.
Figures





References
-
- Ault JG, Rieder CL. Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol. 1994;6:41–49. - PubMed
-
- Blangy A, Arnaud L, Nigg EA. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J Biol Chem. 1997;272:19418–19424. - PubMed
-
- Blumenthal T. Trans-splicing and polycistronic transcription in Caenorhabditis elegans. . Trends Genet. 1995;11:132–136. - PubMed
-
- Boleti H, Karsenti E, Vernos I. Xklp2, a novel Xenopuscentrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

