Effects of ivermectin and midecamycin on ryanodine receptors and the Ca2+-ATPase in sarcoplasmic reticulum of rabbit and rat skeletal muscle
- PMID: 9852316
- PMCID: PMC2269079
- DOI: 10.1111/j.1469-7793.1999.313ae.x
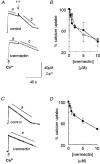
Effects of ivermectin and midecamycin on ryanodine receptors and the Ca2+-ATPase in sarcoplasmic reticulum of rabbit and rat skeletal muscle
Abstract
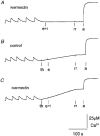
1. Ryanodine receptor (RyR) Ca2+ channels in the sarcoplasmic reticulum (SR) of skeletal muscle are regulated by the 12 kDa FK506- (or rapamycin-) binding protein (FKBP12). Rapamycin can also activate RyR channels with FKBP12 removed, suggesting that compounds with macrocyclic lactone ring structures can directly activate RyRs. Here we tested this hypothesis using two other macrocyclic lactone compounds, ivermectin and midecamycin. 2. Rabbit skeletal RyRs were examined in lipid bilayers. Ivermectin (cis, 0.66-40 microM) activated six of eight native, four of four control-incubated and eleven of eleven FKBP12-'stripped' RyR channels. Midecamycin (cis, 10-30 microM) activated three of four single native channels, six of eight control-incubated channels and six of seven FKBP12-stripped channels. Activity declined when either drug was washed out. 3. Neither ivermectin nor midecamycin removed FKBP12 from RyRs. Western blots of terminal cisternae (TC), incubated for 15 min at 37 C with 40 microM ivermectin or midecamycin, showed normal amounts of FKBP12. In contrast, no FKBP12 was detected after incubation with 40 microM rapamycin. 4. Ivermectin reduced Ca2+ uptake by the SR Ca2+-Mg2+-ATPase. Ca2+ uptake by TC fell to approximately 40% in the presence of ivermectin (10 microM), both with and without 10 microM Ruthenium Red. Ca2+ uptake by longitudinal SR also fell to approximately 40% with 10 microM ivermectin. Midecamycin (10 microM) reduced Ca2+ uptake by TC vesicles to approximately 76% without Ruthenium Red and to approximately 90 % with Ruthenium Red. 5. The rate of rise of extravesicular [Ca2+] increased approximately 2-fold when 10 microM ivermectin was added to TC vesicles that had been partially loaded with Ca2+ and then Ca2+ uptake blocked by 200 nM thapsigargin. Ivermectin also potentiated caffeine-induced Ca2+ release to approximately 140% of control. These increases in Ca2+ release were not seen with midecamycin. 6. Ivermectin, but not midecamycin, reversibly reduced Ca2+ loading in four of six skinned rat extensor digitorum longus (EDL) fibres to approximately 90%, and reversibly increased submaximal caffeine-induced contraction in five of eight fibres by approximately 110% of control. Neither ivermectin nor midecamycin altered twitch or tetanic tension in intact EDL muscle fibres within 20 min of drug addition. 7. The results confirm the hypothesis that compounds with a macrocyclic lactone ring structure can directly activate RyRs. Unexpectedly, ivermectin also reduced Ca2+ uptake into the SR. These effects of ivermectin on SR Ca2+ handling may explain some effects of the macrolide drugs on mammals.
Figures





Similar articles
-
Subconductance states in single-channel activity of skeletal muscle ryanodine receptors after removal of FKBP12.Biophys J. 1997 Jan;72(1):146-62. doi: 10.1016/S0006-3495(97)78654-5. Biophys J. 1997. PMID: 8994600 Free PMC article.
-
Activation and inhibition of skeletal RyR channels by a part of the skeletal DHPR II-III loop: effects of DHPR Ser687 and FKBP12.Biophys J. 1999 Jul;77(1):189-203. doi: 10.1016/S0006-3495(99)76881-5. Biophys J. 1999. PMID: 10388749 Free PMC article.
-
FK506 (tacrolimus) increases halothane-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum.Anesthesiology. 2000 May;92(5):1361-5. doi: 10.1097/00000542-200005000-00026. Anesthesiology. 2000. PMID: 10781282
-
Calcium-gated calcium channels in sarcoplasmic reticulum of rabbit skinned skeletal muscle fibers.J Gen Physiol. 1986 Feb;87(2):289-303. doi: 10.1085/jgp.87.2.289. J Gen Physiol. 1986. PMID: 2419485 Free PMC article. Review.
-
Immunophilins and coupled gating of ryanodine receptors.Curr Top Med Chem. 2003;3(12):1383-91. doi: 10.2174/1568026033451907. Curr Top Med Chem. 2003. PMID: 12871170 Review.
Cited by
-
Effects of the immunosuppressant FK506 on intracellular Ca2+ release and Ca2+ accumulation mechanisms.J Physiol. 2000 Jun 15;525 Pt 3(Pt 3):681-93. doi: 10.1111/j.1469-7793.2000.t01-1-00681.x. J Physiol. 2000. PMID: 10856121 Free PMC article.
-
The inhibition of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase by macrocyclic lactones and cyclosporin A.Biochem J. 2002 Aug 15;366(Pt 1):255-63. doi: 10.1042/BJ20020431. Biochem J. 2002. PMID: 12022919 Free PMC article.
-
FK506-binding proteins 12 and 12.6 (FKBPs) as regulators of cardiac Ryanodine Receptors: Insights from new functional and structural knowledge.Channels (Austin). 2017 Sep 3;11(5):415-425. doi: 10.1080/19336950.2017.1344799. Epub 2017 Jun 21. Channels (Austin). 2017. PMID: 28636428 Free PMC article. Review.
-
Molecular basis for allosteric regulation of the type 2 ryanodine receptor channel gating by key modulators.Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25575-25582. doi: 10.1073/pnas.1914451116. Epub 2019 Dec 2. Proc Natl Acad Sci U S A. 2019. PMID: 31792195 Free PMC article.
-
Ivermectin is a nonselective inhibitor of mammalian P-type ATPases.Naunyn Schmiedebergs Arch Pharmacol. 2010 Feb;381(2):147-52. doi: 10.1007/s00210-009-0483-z. Epub 2009 Dec 30. Naunyn Schmiedebergs Arch Pharmacol. 2010. PMID: 20041321
References
-
- Ahern GP, Junankar PR, Dulhunty AF. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK506. FEBS Letters. 1994;352:369–374. 10.1016/0014-5793(94)01001-3. - DOI - PubMed
-
- Ahern GP, Junankar PR, Dulhunty AF. Ryanodine receptors from rabbit skeletal muscle are reversibly activated by rapamycin. Neuroscience Letters. 1997b;225:81–84. 10.1016/S0304-3940(97)00193-6. - DOI - PubMed
-
- Arena JP, Liu KK, Paress PS, Frazier EG, Cully DF, Mrozik H, Schaeffer JM. The mechanism of action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. Journal of Parasitology. 1995;81:286–294. - PubMed
-
- Bloomquist JR. Toxicology, mode of action and target site-mediated resistance to insecticides acting on chloride channels. Comparitive Biochemistry and Physiology C. 1993;106:301–314. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

