Divalent cation permeability and blockade of Ca2+-permeant non-selective cation channels in rat adrenal zona glomerulosa cells
- PMID: 9852322
- PMCID: PMC2269083
- DOI: 10.1111/j.1469-7793.1999.397ae.x
Divalent cation permeability and blockade of Ca2+-permeant non-selective cation channels in rat adrenal zona glomerulosa cells
Abstract
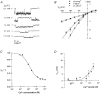
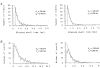
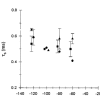
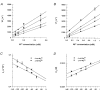
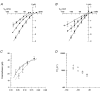
1. The effects of the divalent cations Ca2+, Mg2+ and Ni2+ on unitary Na+ currents through receptor-regulated non-selective cation channels were studied in inside-out and cell-attached patches from rat adrenal zona glomerulosa cells. 2. External Ca2+ caused a concentration-dependent and voltage-independent inhibition of inward Na+ current, exhibiting an IC50 of 1.4 mM. The channel was also Ca2+ permeant and external Ca2+ shifted the reversal potential as expected for a channel exhibiting a constant Ca2+ : Na+ permeability ratio near to 4. 3. External and internal 2 mM Mg2+ caused voltage-dependent inhibition of inward and outward Na+ current, respectively. Modelling Mg2+ as an impermeant fast open channel blocker indicated that external Mg2+ blocked the pore at a single site exhibiting a zero voltage Kd of 5.1 mM for Mg2+ and located 19 % of the distance through the transmembrane electric field from the external surface. Internal Mg2+ blocked the pore at a second site exhibiting a Kd of 1.7 mM for Mg2+ and located 36% of the distance through the transmembrane electric field from the cytosolic surface. 4. External Ni2+ caused a voltage- and concentration-dependent slow blockade of inward Na+ current. Modelling Ni2+ as an impermeant slow open channel blocker indicated that Ni2+ blocked the pore at a single site exhibiting a Kd of 1.09 mM for Ni2+ and located 13.7% of the distance through the transmembrane electric field from the external surface. 5. External 2 mM Mg2+ increased the Kd for external Ni2+ binding to 1.27 mM, consistent with competition for a single binding site. Changing ionic strength did not substantially affect Ni2+ blockade indicating the absence of surface potential under physiological ionic conditions. 6. It is concluded that at least two divalent cation binding sites, separated by a high free energy barrier (the selectivity filter), are located in the pore and contribute to Ca2+ selectivity and permeability of the channel.
Figures
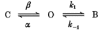


References
-
- Changeux JP, Galzi JL, Devillers-Thiéry A, Bertrand D. The functional architecture of the acetylcholine nicotinic receptor explored by affinity labeling and site-directed mutagenesis. Quarterly Review of Biophysics. 1992;25:395–432. - PubMed
-
- Colquhoun D, Sigworth FJ. Single Channel Recording. New York and London: Plenum Press; 1995. Fitting and statistical analysis of single-channel records; pp. 483–585.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

