Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells
- PMID: 9852330
- PMCID: PMC2269068
- DOI: 10.1111/j.1469-7793.1999.493ae.x
Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells
Abstract
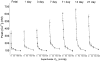
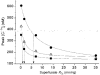
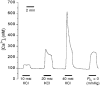
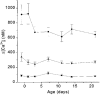
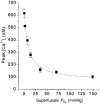
1. Carotid chemoreceptor sensitivity is minimal immediately after birth and increases with postnatal age. In the present study we have investigated the peri- and postnatal developmental time course of [Ca2+]i responses to hypoxia in clusters of type I cells isolated from near-term fetal rats and rats that were 1, 3, 7, 11, 14 and 21 days old, using the Ca2+-sensitive fluoroprobe fura-2. 2. In type I cells from all age groups a graded increase in [Ca2+]i occurred in response to lowering the PO2 from 150 mmHg to 70, 35, 14, 7, 2 and 0 mmHg. The graded [Ca2+]i response to hypoxia was hyperbolic at all ages. 3. Type I cells from rats near-term fetal to 1 day old exhibited small [Ca2+]i responses, mainly to the most severe levels of hypoxia. After day 1, an increase in the [Ca2+]i responses to submaximal hypoxia stimulation resulted in a rightward shift in the O2 response curve. Using the Delta[Ca2+]i between 35 and 2 mmHg PO2 as an index of O2 sensitivity, type I cell O2 sensitivity increased approximately 4- to 5-fold between near-term fetal to 1 day old and 11 to 14 days of age. 4. Exposure to elevated extracellular potassium (10, 20 and 40 mM K+) caused a dose-dependent [Ca2+]i rise in type I cells from all age groups. There were no age-related changes in [Ca2+]i responses to any level of K+ between near-term fetal and 21 days. 5. We conclude that the maximal type I cell [Ca2+]i response to anoxia, as well as the sensitivity to submaximal hypoxic stimulation, of rats aged from near-term fetal to 21 days depends on the level of postnatal maturity. The lack of an age-related increase in the [Ca2+]i response to elevated K+ during the timeframe of maximal development of O2 sensitivity suggests that resetting involves maturation of O2 sensing, rather than non-specific developmental changes in the [Ca2+]i rise resulting from depolarization.
Figures

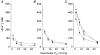

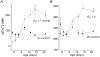
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

