Identification of rhythmically active cells in guinea-pig stomach
- PMID: 9852332
- PMCID: PMC2269070
- DOI: 10.1111/j.1469-7793.1999.515ae.x
Identification of rhythmically active cells in guinea-pig stomach
Abstract
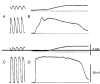
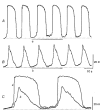
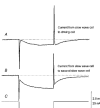
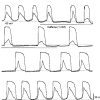
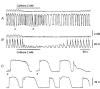
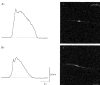
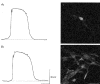
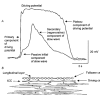
1. When intracellular recordings were made from the antral region of guinea-pig stomach, cells with different patterns of electrical activity were detected. 2. One group of cells, slow-wave cells, generated slow waves which consisted of initial and secondary components. When filled with either Lucifer Yellow or neurobiotin, the cells identified as smooth muscle cells lying in the circular muscle layer. 3. A second group of cells, driving cells, generated large, rapidly rising, potential changes, driving potentials. They had small cell bodies with several processes. With neurobiotin, a network of cells was visualized that resembled c-kit positive interstitial cells of the myenteric region. 4. A third group of cells generated sequences of potential changes which resembled driving potentials but had smaller amplitudes and slow rates of rise. These cells resembled smooth muscle cells lying in the longitudinal muscle layer. 5. When simultaneous recordings were made from the driving and slow-wave cells, driving potentials and slow waves occurred synchronously. Current injections indicated that both cell types were part of a common electrical syncytium. 6. The initial component of slow waves persisted in low concentrations of caffeine, but the secondary component was abolished; higher concentrations shortened the duration of the residual initial component. Driving potentials continued in the presence of low concentrations of caffeine; moderate concentrations of caffeine shortened their duration. 7. Hence three different types of cells were distinguished on the basis of their electrical activity, their responses to caffeine and their structure. These were smooth muscle cells, lying in the longitudinal and circular layers, and interstitial cells in the myenteric region. The observations suggest that interstitial cells initiate slow waves.
Figures




References
-
- Anderson K-E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacological Reviews. 1993;45:253–307. - PubMed
-
- Barajas-Lopez C, Berezin I, Daniel EE, Huizinga JD. Pacemaker activity recorded in interstitial cells of Cajal of the gastrointestinal tract. American Journal of Physiology. 1989;257:C830–835. - PubMed
-
- Bauer AJ, Publicover NG, Sanders KM. Origin and spread of slow waves in canine gastric antral circular muscle. American Journal of Physiology. 1985;249:G800–806. - PubMed
-
- Beny JL, Connat JL. An electron-microscopic study of smooth muscle dye coupling in the pig coronary arteries. Role of gap junctions. Circulation Research. 1992;70:49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

