Nitric oxide-producing islet cells modulate the release of sensory neuropeptides in the rat substantia gelatinosa
- PMID: 9852575
- PMCID: PMC6793334
- DOI: 10.1523/JNEUROSCI.18-24-10375.1998
Nitric oxide-producing islet cells modulate the release of sensory neuropeptides in the rat substantia gelatinosa
Abstract
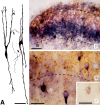
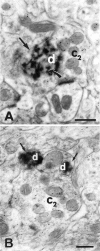
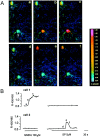
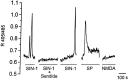
The substantia gelatinosa of the spinal cord (lamina II) is the major site of integration for nociceptive information. Activation of NMDA glutamate receptor, production of nitric oxide (NO), and enhanced release of substance P and calcitonin gene-related peptide (CGRP) from primary afferents are key events in pain perception and central hyperexcitability. By combining reduced nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase histochemistry for NO-producing neurons with immunogold labeling for substance P, CGRP, and glutamate, we show that (1) NO-producing neurons in lamina IIi are islet cells; (2) these neurons rarely form synapses onto peptide-immunoreactive profiles; and (3) NADPH diaphorase-positive dendrites are often in close spatial relationship with peptide-containing terminals and are observed at the periphery of type II glomeruli showing glutamate-immunoreactive central endings. By means of confocal fluorescent microscopy in acute spinal cord slices loaded with the Ca2+ indicator Indo-1, we also demonstrate that (1) NMDA evokes a substantial [Ca2+]i increase in a subpopulation of neurons in laminae I-II, with morphological features similar to those of islet cells; (2) a different neuronal population in laminae I-IIo, unresponsive to NMDA, displays a significant [Ca2+]i increase after slice perfusion with either substance P and the NO donor 3morpholinosydnonimine (SIN-1); and (3) the responses to both substance P and SIN-1 are either abolished or significantly inhibited by the NK1 receptor antagonist sendide. These results provide compelling evidence that glutamate released at type II glomeruli triggers the production of NO in islet cells within lamina IIi after NMDA receptor activation. The release of substance P from primary afferents triggered by newly synthesized NO may play a crucial role in the cellular mechanism leading to spinal hyperexcitability and increased pain perception.
Figures



References
-
- Aimar P, Barajon I, Merighi A. Ultrastructural features and synaptic connections of NADPH-diaphorase positive neurones in the rat spinal dorsal horn and central grey matter. Eur J Anat. 1998;2:27–33.
-
- Aimi Y, Fujimura M, Vincent SR, Kimura H. Localization of NADPH-diaphorase-containing neurons in sensory ganglia of the rat. J Comp Neurol. 1991;306:382–392. - PubMed
-
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980;194:809–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
