Postsynaptic Ca2+ influx mediated by three different pathways during synaptic transmission at a calyx-type synapse
- PMID: 9852578
- PMCID: PMC6793368
- DOI: 10.1523/JNEUROSCI.18-24-10409.1998
Postsynaptic Ca2+ influx mediated by three different pathways during synaptic transmission at a calyx-type synapse
Abstract
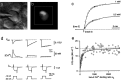
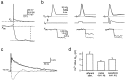
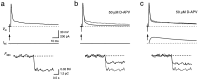
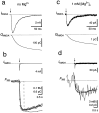
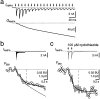
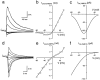
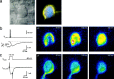
Whole-cell recordings and Ca2+ flux measurements were made at a giant calyx-type synapse in rat brainstem slices to determine the contribution of glutamate receptor (GluR) channels and voltage-dependent Ca2+ channels (VDCCs) to postsynaptic Ca2+ influx during synaptic transmission. A single presynaptic action potential (AP) evoked an EPSP, followed by a single AP. The EPSP-AP sequence caused a postsynaptic Ca2+ influx of approximately 3.0 pC, primarily through VDCCs ( approximately 70%) and NMDA-type (up to 30%) channels but also through AMPA-type (<5%) GluR channels. At -80 mV, the fractional Ca2+ current (Pf) mediated by AMPA receptor (AMPAR) and NMDA receptor (NMDAR) channels was 1.3 and 11-12%, respectively. Simulations of the time course of Ca2+ influx through GluR channels suggested that the small contribution of AMPAR channels occurred only during the first few milliseconds of an EPSP, whereas influx through NMDAR channels dominated later. The NMDAR-mediated Ca2+ influx was localized in regions covered by the presynaptic terminal, whereas the Ca2+ influx mediated by VDCCs was more homogeneously distributed. Because of the temporal and spatial differences, calcium ions entering through the three different pathways are likely to activate different intracellular targets in the postsynaptic cell.
Figures

References
-
- Bito H, Deisseroth K, Tsien RW. Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol. 1997;7:419–429. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
