Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty
- PMID: 9852594
- PMCID: PMC6793358
- DOI: 10.1523/JNEUROSCI.18-24-10579.1998
Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty
Abstract
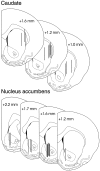
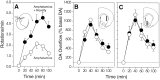
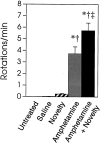
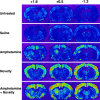
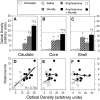
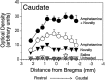
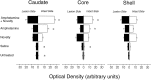
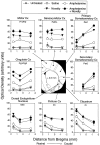
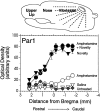
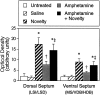
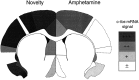
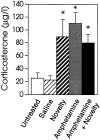
We have shown recently that the psychomotor activating effects of amphetamine in the rat are much greater when this drug is administered in association with environmental novelty than when it is given in a home environment. The main purpose of the present study was to explore the neural basis of this phenomenon. We found, using in situ hybridization of c-fos mRNA, that the pattern of neuronal activation in the cortex, in the caudate, in the shell and core of the nucleus accumbens, and in other subcortical structures was markedly different when amphetamine (2.0 mg/kg, i.p.) was given in association with exposure to environmental novelty relative to when it was given at home. In most brain regions the magnitude of c-fos expression was over two times greater in rats given amphetamine plus novelty than in rats given amphetamine alone. In contrast, an in vivo microdialysis study indicated that environmental novelty did not affect amphetamine-induced dopamine release in either caudate or nucleus accumbens. Furthermore, a unilateral 6-hydroxydopamine lesion of the mesostriatal dopamine system reduced amphetamine- but not novelty-induced c-fos expression. Finally, we found no differences in the amount of corticosterone secreted after exposure to novelty, amphetamine, or both, suggesting that corticosterone does not play a critical role in the ability of novelty to modulate amphetamine-induced psychomotor activation. In conclusion, it seems that environmental novelty alters the neurobiological effects of amphetamine independently of the primary neuropharmacological actions of this drug in the striatum.
Figures

References
-
- Badiani A, Anagnostaras S, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 1995a;117:443–452. - PubMed
-
- Badiani A, Morano MI, Akil H, Robinson TE. Circulating adrenal hormones are not necessary for the development of sensitization to the psychomotor activating effects of amphetamine. Brain Res. 1995b;673:13–24. - PubMed
-
- Badiani A, Browman KE, Robinson TE. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995c;674:291–298. - PubMed
-
- Badiani A, Camp DM, Robinson TE. Enduring enhancement of amphetamine sensitization by drug-associated environmental stimuli. J Pharmacol Exp Ther. 1997;282:787–794. - PubMed
-
- Badiani A, Oates MM, Day HEW, Watson S, Akil H, Robinson TE. Effects of environmental novelty on amphetamine-induced c-fos expression in D1 and D2 striatal neurons. Soc Neurosci Abstr. 1998;24:954.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
