Glutamate inhibits GABA excitatory activity in developing neurons
- PMID: 9852609
- PMCID: PMC6793361
- DOI: 10.1523/JNEUROSCI.18-24-10749.1998
Glutamate inhibits GABA excitatory activity in developing neurons
Abstract
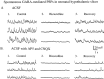
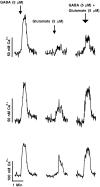
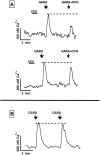
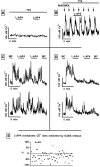
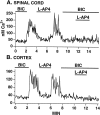
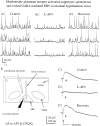
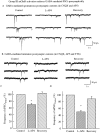
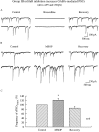
In contrast to the mature brain, in which GABA is the major inhibitory neurotransmitter, in the developing brain GABA can be excitatory, leading to depolarization, increased cytoplasmic calcium, and action potentials. We find in developing hypothalamic neurons that glutamate can inhibit the excitatory actions of GABA, as revealed with fura-2 digital imaging and whole-cell recording in cultures and brain slices. Several mechanisms for the inhibitory role of glutamate were identified. Glutamate reduced the amplitude of the cytoplasmic calcium rise evoked by GABA, in part by activation of group II metabotropic glutamate receptors (mGluRs). Presynaptically, activation of the group III mGluRs caused a striking inhibition of GABA release in early stages of synapse formation. Similar inhibitory actions of the group III mGluR agonist L-AP4 on depolarizing GABA activity were found in developing hypothalamic, cortical, and spinal cord neurons in vitro, suggesting this may be a widespread mechanism of inhibition in neurons throughout the developing brain. Antagonists of group III mGluRs increased GABA activity, suggesting an ongoing spontaneous glutamate-mediated inhibition of excitatory GABA actions in developing neurons. Northern blots revealed that many mGluRs were expressed early in brain development, including times of synaptogenesis. Together these data suggest that in developing neurons glutamate can inhibit the excitatory actions of GABA at both presynaptic and postsynaptic sites, and this may be one set of mechanisms whereby the actions of two excitatory transmitters, GABA and glutamate, do not lead to runaway excitation in the developing brain. In addition to its independent excitatory role that has been the subject of much attention, our data suggest that glutamate may also play an inhibitory role in modulating the calcium-elevating actions of GABA that may affect neuronal migration, synapse formation, neurite outgrowth, and growth cone guidance during early brain development.
Figures

References
-
- Barbin G, Pollard H, Gaiarsa JL, Ben-Ari Y. Involvement of GABA-A receptors in the outgrowth of cultured hippocampal neurones. Neurosci Lett. 1993;152:150–154. - PubMed
-
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. Gamma-aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. - PubMed
-
- Bonci A, Grillner P, Siniscalchi A, Mercuri NB, Bernardi G. Glutamate metabotropic receptor agonists depress excitatory and inhibitory transmission on rat mesencephalic principal neurons. Eur J Neurosci. 1997;9:2359–2369. - PubMed
-
- Chen G, van den Pol AN. Coexpression of multiple metabotropic glutamate receptors in axon terminals of single suprachiasmatic nucleus neurons. J Neurophysiol. 1998;80:1932–1938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
