Genetic control of natural killing and in vivo tumor elimination by the Chok locus
- PMID: 9858511
- PMCID: PMC2212436
- DOI: 10.1084/jem.188.12.2243
Genetic control of natural killing and in vivo tumor elimination by the Chok locus
Abstract
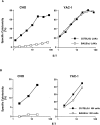
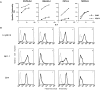
The molecular mechanisms underlying target recognition during natural killing are not well understood. One approach to dissect the complexities of natural killer (NK) cell recognition is through exploitation of genetic differences among inbred mouse strains. In this study, we determined that interleukin 2-activated BALB/c-derived NK cells could not lyse Chinese hamster ovary (CHO) cells as efficiently as C57BL/6-derived NK cells, despite equivalent capacity to kill other targets. This strain-determined difference was also exhibited by freshly isolated NK cells, and was determined to be independent of host major histocompatibility haplotype. Furthermore, CHO killing did not correlate with expression of NK1.1 or 2B4 activation molecules. Genetic mapping studies revealed linkage between the locus influencing CHO killing, termed Chok, and loci encoded within the NK gene complex (NKC), suggesting that Chok encodes an NK cell receptor specific for CHO cells. In vivo assays recapitulated the in vitro data, and both studies determined that Chok regulates an NK perforin-dependent cytotoxic process. These results may have implications for the role of NK cells in xenograft rejection. Our genetic analysis suggests Chok is a single locus that affects NK cell-mediated cytotoxicity similar to other NKC loci that also regulate the complex activity of NK cells.
Figures




Similar articles
-
The natural killer gene complex genetic locus Chok encodes Ly-49D, a target recognition receptor that activates natural killing.Proc Natl Acad Sci U S A. 1999 May 25;96(11):6330-5. doi: 10.1073/pnas.96.11.6330. Proc Natl Acad Sci U S A. 1999. PMID: 10339587 Free PMC article.
-
A ligand for the murine NK activation receptor Ly-49D: activation of tolerized NK cells from beta 2-microglobulin-deficient mice.J Immunol. 2002 Jul 1;169(1):126-36. doi: 10.4049/jimmunol.169.1.126. J Immunol. 2002. PMID: 12077237
-
Close genetic linkage of Chok with the NKC-linked loci Cd94, Ly49, and Cmv1 on mouse chromosome 6.Immunogenetics. 1999 Sep;49(10):906-8. doi: 10.1007/s002510050572. Immunogenetics. 1999. PMID: 10436186 No abstract available.
-
Physiologic functions of activating natural killer (NK) complex-encoded receptors on NK cells.Immunol Rev. 2001 Jun;181:126-37. doi: 10.1034/j.1600-065x.2001.1810110.x. Immunol Rev. 2001. PMID: 11513134 Review.
-
Ontogeny and differentiation of murine natural killer cells and their receptors.Curr Top Microbiol Immunol. 1998;230:161-90. doi: 10.1007/978-3-642-46859-9_11. Curr Top Microbiol Immunol. 1998. PMID: 9586356 Review. No abstract available.
Cited by
-
Ligand dimensions are important in controlling NK-cell responses.Eur J Immunol. 2010 Jul;40(7):2050-9. doi: 10.1002/eji.201040335. Eur J Immunol. 2010. PMID: 20432238 Free PMC article.
-
Requirement for membrane lymphotoxin in natural killer cell development.Proc Natl Acad Sci U S A. 1999 May 25;96(11):6336-40. doi: 10.1073/pnas.96.11.6336. Proc Natl Acad Sci U S A. 1999. PMID: 10339588 Free PMC article.
-
Enhanced protection of C57 BL/6 vs Balb/c mice to melanoma liver metastasis is mediated by NK cells.Oncoimmunology. 2017 Dec 26;7(4):e1409929. doi: 10.1080/2162402X.2017.1409929. eCollection 2018. Oncoimmunology. 2017. PMID: 29632723 Free PMC article.
-
Murine trophoblast cells induce NK cell interferon-gamma production through KLRK1.Biol Reprod. 2010 Sep;83(3):404-14. doi: 10.1095/biolreprod.110.084509. Epub 2010 May 19. Biol Reprod. 2010. PMID: 20484740 Free PMC article.
-
Endostatin gene therapy enhances the efficacy of IL-2 in suppressing metastatic renal cell carcinoma in mice.Cancer Immunol Immunother. 2010 Sep;59(9):1357-65. doi: 10.1007/s00262-010-0865-6. Epub 2010 May 20. Cancer Immunol Immunother. 2010. PMID: 20490489 Free PMC article.
References
-
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. - PubMed
-
- Trinchieri G. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin Immunol. 1995;7:83–88. - PubMed
-
- Bancroft GJ. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. - PubMed
-
- Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

