The central role of CD4(+) T cells in the antitumor immune response
- PMID: 9858522
- PMCID: PMC2212434
- DOI: 10.1084/jem.188.12.2357
The central role of CD4(+) T cells in the antitumor immune response
Abstract
The induction of optimal systemic antitumor immunity involves the priming of both CD4(+) and CD8(+) T cells specific for tumor-associated antigens. The role of CD4(+) T helper cells (Th) in this response has been largely attributed to providing regulatory signals required for the priming of major histocompatibility complex class I restricted CD8(+) cytolytic T lymphocytes, which are thought to serve as the dominant effector cell mediating tumor killing. However, analysis of the effector phase of tumor rejection induced by vaccination with irradiated tumor cells transduced to secrete granulocyte/macrophage colony-stimulating factor indicates a far broader role for CD4(+) T cells in orchestrating the host response to tumor. This form of immunization leads to the simultaneous induction of Th1 and Th2 responses, both of which are required for maximal systemic antitumor immunity. Cytokines produced by these CD4(+) T cells activate eosinophils as well as macrophages that produce both superoxide and nitric oxide. Both of these cell types then collaborate within the site of tumor challenge to cause its destruction.
Figures



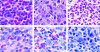

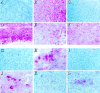
References
-
- Pardoll DM. Tumour antigens. A new look for the 1990s. Nature. 1994;369:357. - PubMed
-
- Kawakami Y, Rosenberg SA. Human tumor antigens recognized by T-cells. Immunol Res. 1997;16:313–339. - PubMed
-
- Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. - PubMed
-
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony–stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

