The yeast telomere length counting machinery is sensitive to sequences at the telomere-nontelomere junction
- PMID: 9858529
- PMCID: PMC83863
- DOI: 10.1128/MCB.19.1.31
The yeast telomere length counting machinery is sensitive to sequences at the telomere-nontelomere junction
Abstract
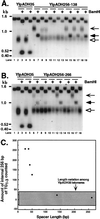
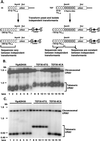
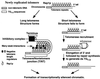
Saccharomyces cerevisiae telomeres consist of a continuous 325 +/- 75-bp tract of the heterogeneous repeat TG1-3 which contains irregularly spaced, high-affinity sites for the protein Rap1p. Yeast cells monitor or count the number of telomeric Rap1p molecules in a negative feedback mechanism which modulates telomere length. To investigate the mechanism by which Rap1p molecules are counted, the continuous telomeric TG1-3 sequences were divided into internal TG1-3 sequences and a terminal tract separated by nontelomeric spacers of different lengths. While all of the internal sequences were counted as part of the terminal tract across a 38-bp spacer, a 138-bp disruption completely prevented the internal TG1-3 sequences from being considered part of the telomere and defined the terminal tract as a discrete entity separate from the subtelomeric sequences. We also used regularly spaced arrays of six Rap1p sites internal to the terminal TG1-3 repeats to show that each Rap1p molecule was counted as about 19 bp of TG1-3 in vivo and that cells could count Rap1p molecules with different spacings between tandem sites. As previous in vitro experiments had shown that telomeric Rap1p sites occur about once every 18 bp, all Rap1p molecules at the junction of telomeric and nontelomeric chromatin (the telomere-nontelomere junction) must participate in telomere length measurement. The conserved arrangement of these six Rap1p molecules at the telomere-nontelomere junction in independent transformants also caused the elongated TG1-3 tracts to be maintained at nearly identical lengths, showing that sequences at the telomere-nontelomere junction had an effect on length regulation. These results can be explained by a model in which telomeres beyond a threshold length form a folded structure that links the chromosome terminus to the telomere-nontelomere junction and prevents telomere elongation.
Figures




References
-
- Aparicio O M, Gottschling D E. Overcoming telomeric silencing: a transactivator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994;8:1133–1146. - PubMed
-
- Brun C, Marcand S, Gilson E. Proteins that bind to double-stranded regions of telomeric DNA. Trends Cell Biol. 1997;7:317–324. - PubMed
-
- Conrad M N, Wright J H, Wolf A J, Zakian V A. RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell. 1990;63:739–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
