Transcriptional and posttranscriptional silencing of rodent alpha1(I) collagen by a homologous transcriptionally self-silenced transgene
- PMID: 9858551
- PMCID: PMC83885
- DOI: 10.1128/MCB.19.1.274
Transcriptional and posttranscriptional silencing of rodent alpha1(I) collagen by a homologous transcriptionally self-silenced transgene
Abstract
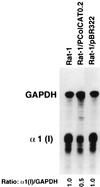
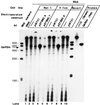
Transient transfection of rodent fibroblasts with plasmids carrying a full-size pro-alpha1(I) collagen gene (pWTC1) results in rapid reduction of the endogenous transcripts by >90%, while the transgene mRNA is undetectable. Using deletion constructs, two adjacent 5' noncoding regions of the gene are identified as causing transcriptional silencing of the endogene in normal and v-fos-transformed cells but not in nontumorigenic revertants, which show partial relief from v-fos transformation-induced alpha1(I) gene suppression. The 3' end of the transgene carries an additional element(s), causing posttranscriptional silencing of the endogene in all cells including the revertant. Data indicate that the transgenes are transcriptionally self-silenced. Genome-integrated transgenes that are transcriptionally active also allow expression of the endogene, suggesting gene activation by chromosomal factors missing in pWTC1. Silencing is not regulated by antisense RNA. Silencing of the endogenous pro-alpha1(I) collagen gene is not linked to the level of transgene expression.
Figures





References
-
- Bahramian M B, Zarbl H. Direct gene quantitations by PCR reveal differential accumulation of ectopic enzyme in Rat-1 cells, v-fos transformants and revertants. PCR Methods Appl. 1994;4:145–153. - PubMed
-
- Bahramian, M. B., C. D. Hoemann, and H. Zarbl. Unpublished data.
-
- Baulcombe D C, English J J. Ectopic pairing of homologous DNA and posttranscriptional gene silencing in transgenic plants. Curr Opin Biotechnol. 1996;7:173–180.
-
- Bingham P M. Cosuppression comes to the animals. Cell. 1997;90:385–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
