Cyclin E2, a novel G1 cyclin that binds Cdk2 and is aberrantly expressed in human cancers
- PMID: 9858585
- PMCID: PMC83919
- DOI: 10.1128/MCB.19.1.612
Cyclin E2, a novel G1 cyclin that binds Cdk2 and is aberrantly expressed in human cancers
Abstract
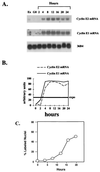
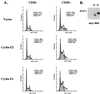
A novel cyclin gene was discovered by searching an expressed sequence tag database with a cyclin box profile. The human cyclin E2 gene encodes a 404-amino-acid protein that is most closely related to cyclin E. Cyclin E2 associates with Cdk2 in a functional kinase complex that is inhibited by both p27(Kip1) and p21(Cip1). The catalytic activity associated with cyclin E2 complexes is cell cycle regulated and peaks at the G1/S transition. Overexpression of cyclin E2 in mammalian cells accelerates G1, demonstrating that cyclin E2 may be rate limiting for G1 progression. Unlike cyclin E1, which is expressed in most proliferating normal and tumor cells, cyclin E2 levels were low to undetectable in nontransformed cells and increased significantly in tumor-derived cells. The discovery of a novel second cyclin E family member suggests that multiple unique cyclin E-CDK complexes regulate cell cycle progression.
Figures






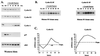
References
-
- Catzavelos C, Bhattacharya N, Ung Y, Wilson J, Roncari L, Sandhu C, Shaw R, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard K, Slingerland J. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227–230. - PubMed
-
- Clurman B E, Sheaff R, Thress K, Groudine M, Roberts J M. Cdk2-dependent cyclin E ubiquitination. Genes Dev. 1996;10:1979–1990. - PubMed
-
- Ethier S P, Mahacek M L, Gullick W J, Frank T S, Weber B L. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites. Cancer Res. 1993;53:627–635. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
