Temperature, template topology, and factor requirements of archaeal transcription
- PMID: 9860949
- PMCID: PMC28023
- DOI: 10.1073/pnas.95.26.15218
Temperature, template topology, and factor requirements of archaeal transcription
Abstract
Although Archaea are prokaryotic and resemble Bacteria morphologically, their transcription apparatus is remarkably similar to those of eukaryotic cell nuclei. Because some Archaea exist in environments with temperatures of around 100 degreesC, they are likely to have evolved unique strategies for transcriptional control. Here, we investigate the effects of temperature and DNA template topology in a thermophilic archaeal transcription system. Significantly, and in marked contrast with characterized eucaryal systems, archaeal DNA template topology has negligible effect on transcription levels at physiological temperatures using highly purified polymerase and recombinant transcription factors. Furthermore, archaeal transcription does not require hydrolysis of the beta-gamma phosphoanhydride bond of ATP. However, at lower temperatures, negatively supercoiled templates are transcribed more highly than those that are positively supercoiled. Notably, the block to transcription on positively supercoiled templates at lowered temperatures is at the level of polymerase binding and promoter opening. These data imply that Archaea do not possess a functional homologue of transcription factor TFIIH, and that for the promoters studied, transcription is mediated by TATA box-binding protein, transcription factor TFB, and RNA polymerase alone. Furthermore, they suggest that the reduction of plasmid linking number by hyperthermophilic Archaea in vivo in response to cold shock is a mechanism to maintain gene expression under these adverse circumstances.
Figures

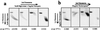


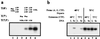

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

