Novel role of phosphorylation in Fe-S cluster stability revealed by phosphomimetic mutations at Ser-138 of iron regulatory protein 1
- PMID: 9860952
- PMCID: PMC28026
- DOI: 10.1073/pnas.95.26.15235
Novel role of phosphorylation in Fe-S cluster stability revealed by phosphomimetic mutations at Ser-138 of iron regulatory protein 1
Abstract
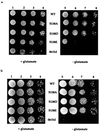
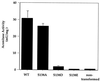
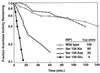
Animals regulate iron metabolism largely through the action of the iron regulatory proteins (IRPs). IRPs modulate mRNA utilization by binding to iron-responsive elements (IRE) in the 5' or 3' untranslated region of mRNAs encoding proteins involved in iron homeostasis or energy production. IRP1 is also the cytosolic isoform of aconitase. The activities of IRP1 are mutually exclusive and are modulated through the assembly/disassembly of its [4Fe-4S] cluster, reversibly converting it between an IRE-binding protein and cytosolic aconitase. IRP1 is also phosphoregulated by protein kinase C, but the mechanism by which phosphorylation posttranslationally increases IRE binding activity has not been fully defined. To investigate this, Ser-138 (S138), a PKC phosphorylation site, was mutated to phosphomimetic glutamate (S138E), aspartate (S138D), or nonphosphorylatable alanine (S138A). The S138E IRP1 mutant and, to a lesser extent, the S138D IRP1 mutant were impaired in aconitase function in yeast when grown aerobically but not when grown anaerobically. Purified wild-type and mutant IRP1s could be reconstituted to active aconitases anaerobically. However, when exposed to oxygen, the [4Fe-4S] cluster of the S138D and S138E mutants decayed 5-fold and 20-fold faster, respectively, than was observed for wild-type IRP1. Our findings suggest that stability of the Fe-S cluster of IRP1 can be regulated by phosphorylation and reveal a mechanism whereby the balance between the IRE binding and [4Fe-4S] forms of IRP1 can be modulated independently of cellular iron status. Furthermore, our results show that IRP1 can function as an oxygen-modulated posttranscriptional regulator of gene expression.
Figures
References
-
- Crichton R R. Inorganic Biochemistry of Iron Metabolism. New York: Ellis Horwood; 1991.
-
- Halliwell B, Gutteridge J M C. Arch Biochem Biophys. 1986;246:501–514. - PubMed
-
- Williams R J P. In: Iron Transport and Storage. Ponka P, Schulman H M, Woodworth R C, editors. Boca Raton, FL: CRC; 1990. pp. 1–16.
-
- Eisenstein R S, Kennedy M C, Beinert H. In: Metal Ions in Gene Regulation. Silver S, Walden W, editors. New York: International Thomson; 1997. pp. 157–216.
-
- Hentze M W, Caughman S W, Rouault T A, Barriocanal J G, Dancis A, Harford J B, Klausner R D. Science. 1987;238:1570–1573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

