A model for amplification of hair-bundle motion by cyclical binding of Ca2+ to mechanoelectrical-transduction channels
- PMID: 9860967
- PMCID: PMC28041
- DOI: 10.1073/pnas.95.26.15321
A model for amplification of hair-bundle motion by cyclical binding of Ca2+ to mechanoelectrical-transduction channels
Abstract
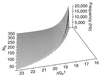
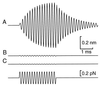
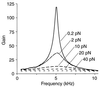
Amplification of auditory stimuli by hair cells augments the sensitivity of the vertebrate inner ear. Cell-body contractions of outer hair cells are thought to mediate amplification in the mammalian cochlea. In vertebrates that lack these cells, and perhaps in mammals as well, active movements of hair bundles may underlie amplification. We have evaluated a mathematical model in which amplification stems from the activity of mechanoelectrical-transduction channels. The intracellular binding of Ca2+ to channels is posited to promote their closure, which increases the tension in gating springs and exerts a negative force on the hair bundle. By enhancing bundle motion, this force partially compensates for viscous damping by cochlear fluids. Linear stability analysis of a six-state kinetic model reveals Hopf bifurcations for parameter values in the physiological range. These bifurcations signal conditions under which the system's behavior changes from a damped oscillatory response to spontaneous limit-cycle oscillation. By varying the number of stereocilia in a bundle and the rate constant for Ca2+ binding, we calculate bifurcation frequencies spanning the observed range of auditory sensitivity for a representative receptor organ, the chicken's cochlea. Simulations using prebifurcation parameter values demonstrate frequency-selective amplification with a striking compressive nonlinearity. Because transduction channels occur universally in hair cells, this active-channel model describes a mechanism of auditory amplification potentially applicable across species and hair-cell types.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

