Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2
- PMID: 9860971
- PMCID: PMC28045
- DOI: 10.1073/pnas.95.26.15345
Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2
Abstract
The ability to sense orientation relative to gravity requires dense particles, called otoconia, which are localized in the vestibular macular organs. In mammals, otoconia are composed of proteins (otoconins) and calcium carbonate crystals in a calcite lattice. Little is known about the mechanisms that regulate otoconial biosynthesis. To begin to elucidate these mechanisms, we have partially sequenced and cloned the major protein component of murine otoconia, otoconin-90 (OC90). The amino acid sequence identified an orphan chimeric human cDNA. Because of its similarity to secretory phospholipase A2 (sPLA2), this gene was referred to as PLA2-like (PLA2L) and enabled the identification of human Oc90. Partial murine cDNA and genomic clones were isolated and shown to be specifically expressed in the developing mouse otocyst. The mature mouse OC90 is composed of 453 residues and contains two domains homologous to sPLA2. The cloning of Oc90 will allow an examination of the role of this protein in otoconial biosynthesis and in diseases that affect the vestibular system.
Figures




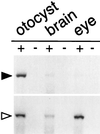

References
-
- Lim D J. Birth Defects. 1980;16:111–146. - PubMed
-
- Mann S, Parker S B, Ross M D, Skarnulis A J, Williams R J. Proc R Soc London B. 1983;218:415–424. - PubMed
-
- Ross M D, Pote K G, Perini F. In: Auditory Biochemistry. Drescher D G, editor. Springfield, IL: Thomas; 1985. pp. 500–514.
-
- Ross M D, Pote K G. Philos Trans R Soc London B. 1984;304:445–452. - PubMed
-
- Carlstrom D. Biol Bull (Woods Hole, Mass) 1963;125:441–463.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

