Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II
- PMID: 9860997
- PMCID: PMC28071
- DOI: 10.1073/pnas.95.26.15496
Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II
Abstract
The classically recognized functions of the renin-angiotensin system are mediated by type 1 (AT1) angiotensin receptors. Whereas man possesses a single AT1 receptor, there are two AT1 receptor isoforms in rodents (AT1A and AT1B) that are products of separate genes (Agtr1a and Agtr1b). We have generated mice lacking AT1B (Agtr1b -/-) and both AT1A and AT1B receptors (Agtr1a -/-Agtr1b -/-). Agtr1b -/- mice are healthy, without an abnormal phenotype. In contrast, Agtr1a -/-Agtr1b -/- mice have diminished growth, vascular thickening within the kidney, and atrophy of the inner renal medulla. This phenotype is virtually identical to that seen in angiotensinogen-deficient (Agt-/-) and angiotensin-converting enzyme-deficient (Ace -/-) mice that are unable to synthesize angiotensin II. Agtr1a -/-Agtr1b -/- mice have no systemic pressor response to infusions of angiotensin II, but they respond normally to another vasoconstrictor, epinephrine. Blood pressure is reduced substantially in the Agtr1a -/- Agtr1b -/- mice and following administration of an angiotensin converting enzyme inhibitor, their blood pressure increases paradoxically. We suggest that this is a result of interruption of AT2-receptor signaling. In summary, our studies suggest that both AT1 receptors promote somatic growth and maintenance of normal kidney structure. The absence of either of the AT1 receptor isoforms alone can be compensated in varying degrees by the other isoform. These studies reaffirm and extend the importance of AT1 receptors to mediate physiological functions of the renin-angiotensin system.
Figures
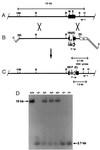




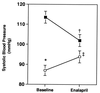
References
-
- Griendling K, Lassegue B, Alexander R. Annu Rev Pharmacol Toxicol. 1996;36:281–306. - PubMed
-
- Timmermans P, Wong P, Chiu A, Herblin W, Benfield P, Carini D, Lee R, Wexler R, Saye J, Smith R. Pharmacol Rev. 1993;45:205–251. - PubMed
-
- Sasamura H, Hein L, Krieger J, Pratt R, Kobilka B, Dzau V. Biochem Biophys Res Comm. 1992;185:253–259. - PubMed
-
- MacTaggart T, Ito M, Smithies O, John S. Mamm Genome. 1997;8(4):294–295. - PubMed
-
- Burson J, Aguilera G, Gross K, Sigmund C. Am J Physiol. 1994;267:E260–E267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

