The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway
- PMID: 9861013
- PMCID: PMC28087
- DOI: 10.1073/pnas.95.26.15587
The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway
Abstract
The PTEN/MMAC1 phosphatase is a tumor suppressor gene implicated in a wide range of human cancers. Here we provide biochemical and functional evidence that PTEN/MMAC1 acts a negative regulator of the phosphoinositide 3-kinase (PI3-kinase)/Akt pathway. PTEN/MMAC1 impairs activation of endogenous Akt in cells and inhibits phosphorylation of 4E-BP1, a downstream target of the PI3-kinase/Akt pathway involved in protein translation, whereas a catalytically inactive, dominant negative PTEN/MMAC1 mutant enhances 4E-BP1 phosphorylation. In addition, PTEN/MMAC1 represses gene expression in a manner that is rescued by Akt but not PI3-kinase. Finally, higher levels of Akt activation are observed in human prostate cancer cell lines and xenografts lacking PTEN/MMAC1 expression when compared with PTEN/MMAC1-positive prostate tumors or normal prostate tissue. Because constitutive activation of either PI3-kinase or Akt is known to induce cellular transformation, an increase in the activation of this pathway caused by mutations in PTEN/MMAC1 provides a potential mechanism for its tumor suppressor function.
Figures
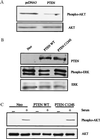

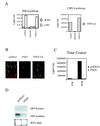
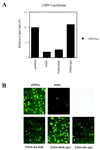

References
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
-
- Steck P A, Pershouse M A, Jasser S A, Yung W K A, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
-
- Teng D H-F, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen K L, Vinson V L, Gumpper K L, et al. Cancer Res. 1998;57:5221–5225. - PubMed
-
- Liaw D, Marsh D J, Li J, Dahia P L M, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

