Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function
- PMID: 9861016
- PMCID: PMC28090
- DOI: 10.1073/pnas.95.26.15603
Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function
Abstract
During the aging process, mammals lose up to a third of their skeletal muscle mass and strength. Although the mechanisms underlying this loss are not entirely understood, we attempted to moderate the loss by increasing the regenerative capacity of muscle. This involved the injection of a recombinant adeno-associated virus directing overexpression of insulin-like growth factor I (IGF-I) in differentiated muscle fibers. We demonstrate that the IGF-I expression promotes an average increase of 15% in muscle mass and a 14% increase in strength in young adult mice, and remarkably, prevents aging-related muscle changes in old adult mice, resulting in a 27% increase in strength as compared with uninjected old muscles. Muscle mass and fiber type distributions were maintained at levels similar to those in young adults. We propose that these effects are primarily due to stimulation of muscle regeneration via the activation of satellite cells by IGF-I. This supports the hypothesis that the primary cause of aging-related impairment of muscle function is a cumulative failure to repair damage sustained during muscle utilization. Our results suggest that gene transfer of IGF-I into muscle could form the basis of a human gene therapy for preventing the loss of muscle function associated with aging and may be of benefit in diseases where the rate of damage to skeletal muscle is accelerated.
Figures
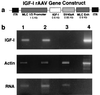
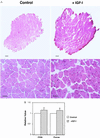

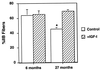
References
-
- Lamberts S W J, van den Beld A W, van der Lely A-J. Science. 1997;278:419–424. - PubMed
-
- Tzankoff S P, Norris A H. J Appl Physiol. 1977;43:1001–1006. - PubMed
-
- Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Acta Physiol Scand. 1982;115:125–134. - PubMed
-
- Larrson L, Edstrom L. J Neurol Sci. 1986;76:69–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

