Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target
- PMID: 9861018
- PMCID: PMC28092
- DOI: 10.1073/pnas.95.26.15613
Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target
Abstract
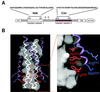
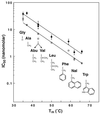
Synthetic C peptides, corresponding to the C helix of the HIV type 1 (HIV-1) gp41 envelope protein, are potent inhibitors of HIV-1 membrane fusion. One such peptide is in clinical trials. The crystal structure of the gp41 core, in its proposed fusion-active conformation, is a trimer of helical hairpins in which three C helices pack against a central coiled coil. Each C helix shows especially prominent contacts with one of three symmetry-related, hydrophobic cavities on the surface of the coiled coil. We show that the inhibitory activity of the C peptide C34 depends on its ability to bind to this coiled-coil cavity. Moreover, examining a series of C34 peptide variants with modified cavity-binding residues, we find a linear relationship between the logarithm of the inhibitory potency and the stability of the corresponding helical-hairpin complexes. Our results provide strong evidence that this coiled-coil cavity is a good drug target and clarify the mechanism of C peptide inhibition. They also suggest simple, quantitative assays for the identification and evaluation of analogous inhibitors of HIV-1 entry.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

