Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice
- PMID: 9861026
- PMCID: PMC28100
- DOI: 10.1073/pnas.95.26.15659
Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice
Abstract
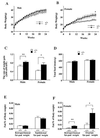
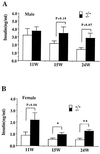
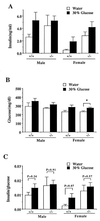
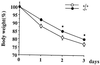
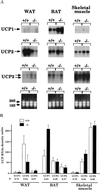
To elucidate the role of neuropeptide Y (NPY)-Y1 receptor (Y1-R) in food intake, energy expenditure, and other possible functions, we have generated Y1-R-deficient mice (Y1-R-/-) by gene targeting. Contrary to our hypothesis that the lack of NPY signaling via Y1-R would result in impaired feeding and weight loss, Y1-R-/- mice showed a moderate obesity and mild hyperinsulinemia without hyperphagia. Although there was some variation between males and females, typical characteristics of Y1-R-/- mice include: greater body weight (females more than males), an increase in the weight of white adipose tissue (WAT) (approximately 4-fold in females), an elevated basal level of plasma insulin (approximately 2-fold), impaired insulin secretion in response to glucose administration, and a significant changes in mitochondrial uncoupling protein (UCP) gene expression (up-regulation of UCP1 in brown adipose tissue and down-regulation of UCP2 in WAT). These results suggest either that the Y1-R in the hypothalamus is not a key molecule in the leptin/NPY pathway, which controls feeding behavior, or that its deficiency is compensated by other receptors, such as NPY-Y5 receptor. We believe that the mild obesity found in Y1-R-/- mice (especially females) was caused by the impaired control of insulin secretion and/or low energy expenditure, including the lowered expression of UCP2 in WAT. This model will be useful for studying the mechanism of mild obesity and abnormal insulin metabolism in noninsulin-dependent diabetes mellitus.
Figures

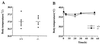
References
-
- Dumont Y, Martel J C, Fournier A, St. Pierre S, Quirion R. Prog Neurobiol. 1992;38:125–167. - PubMed
-
- Wahlestedt C, Reis D J. Annu Rev Pharmacol Toxicol. 1993;32:309–352. - PubMed
-
- Leibowitz S F. Brain Res Bull. 1991;27:333–337. - PubMed
-
- Wahlestedt C, Merlo Pich E, Koob G F, Yee F, Heeilig M. Science. 1993;259:528–530. - PubMed
-
- McDermott B J, Millar B C. Cardiovasc Res. 1993;27:893–905. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

