Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia
- PMID: 9861045
- PMCID: PMC28119
- DOI: 10.1073/pnas.95.26.15769
Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia
Abstract
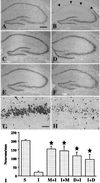
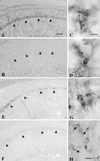
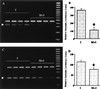
Ischemic stroke is the most common life-threatening neurological disease and has limited therapeutic options. One component of ischemic neuronal death is inflammation. Here we show that doxycycline and minocycline, which are broad-spectrum antibiotics and have antiinflammatory effects independent of their antimicrobial activity, protect hippocampal neurons against global ischemia in gerbils. Minocycline increased the survival of CA1 pyramidal neurons from 10.5% to 77% when the treatment was started 12 h before ischemia and to 71% when the treatment was started 30 min after ischemia. The survival with corresponding pre- and posttreatment with doxycycline was 57% and 47%, respectively. Minocycline prevented completely the ischemia-induced activation of microglia and the appearance of NADPH-diaphorase reactive cells, but did not affect induction of glial acidic fibrillary protein, a marker of astrogliosis. Minocycline treatment for 4 days resulted in a 70% reduction in mRNA induction of interleukin-1beta-converting enzyme, a caspase that is induced in microglia after ischemia. Likewise, expression of inducible nitric oxide synthase mRNA was attenuated by 30% in minocycline-treated animals. Our results suggest that lipid-soluble tetracyclines, doxycycline and minocycline, inhibit inflammation and are neuroprotective against ischemic stroke, even when administered after the insult. Tetracycline derivatives may have a potential use also as antiischemic compounds in humans.
Figures

References
-
- Klein N C, Cunha B A. Med Clin N Am. 1995;79:789–801. - PubMed
-
- Cunha B A, Sibley C, Ristuccia P A. Ther Drug Monit. 1982;4:115–130. - PubMed
-
- Sande M A, Mandell G L. In: The Pharmacological Basis of Therapeutics. Goodman L S, Gilman A, editors. New York: Macmillan; 1985. pp. 1170–1192.
-
- Kramer P A, Chapron D J, Benson J, Mercik S A. Clin Pharmacol Ther. 1978;23:467–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

