Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose
- PMID: 9861057
- PMCID: PMC28131
- DOI: 10.1073/pnas.95.26.15837
Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose
Abstract
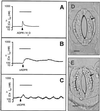
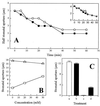
Abscisic acid (ABA) is a plant hormone involved in the response of plants to reduced water availability. Reduction of guard cell turgor by ABA diminishes the aperture of the stomatal pore and thereby contributes to the ability of the plant to conserve water during periods of drought. Previous work has demonstrated that cytosolic Ca2+ is involved in the signal transduction pathway that mediates the reduction in guard cell turgor elicited by ABA. Here we report that ABA uses a Ca2+-mobilization pathway that involves cyclic adenosine 5'-diphosphoribose (cADPR). Microinjection of cADPR into guard cells caused reductions in turgor that were preceded by increases in the concentration of free Ca2+ in the cytosol. Patch clamp measurements of isolated guard cell vacuoles revealed the presence of a cADPR-elicited Ca2+-selective current that was inhibited at cytosolic Ca2+ >/= 600 nM. Furthermore, microinjection of the cADPR antagonist 8-NH2-cADPR caused a reduction in the rate of turgor loss in response to ABA in 54% of cells tested, and nicotinamide, an antagonist of cADPR production, elicited a dose-dependent block of ABA-induced stomatal closure. Our data provide definitive evidence for a physiological role for cADPR and illustrate one mechanism of stimulus-specific Ca2+ mobilization in higher plants. Taken together with other recent data [Wu, Y., Kuzma, J., Marechal, E., Graeff, R., Lee, H. C., Foster, R. & Chua, N.-H. (1997) Science 278, 2126-2130], these results establish cADPR as a key player in ABA signal transduction pathways in plants.
Figures
References
-
- Mittelheuser C J, Van Steveninck Nature (London) 1969;221:281–282.
-
- MacRobbie E A C. J Exp Bot. 1981;32:563–572.
-
- McAinsh M R, Brownlee C, Hetherington A M. Nature (London) 1990;343:186–188.
-
- MacRobbie E A C. J Exp Bot. 1997;48:515–528. - PubMed
-
- Leung J, Giraudat J. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

