Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients
- PMID: 9862244
- PMCID: PMC1873796
- DOI: 10.1046/j.1365-2125.1998.00830.x
Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients
Abstract
Aims: To investigate the pharmacokinetic and pharmacodynamic properties of artemether and benflumetol in a fixed combination tablet (CGP 56697) and to offer an explanation for the lower than expected cure rate in a Thai clinical trial.
Methods: Two hundred and sixty patients were enrolled into a randomized, double-blind, parallel group, dose-finding trial. CGP 56697 was given orally, either as: A, 4 x 4 tablets over 48 h; B, 4 x 2 tablets over 48 h or C, 3 x 4 tablets over 24 h. Each tablet contained artemether 20 mg amd benflumetol 120 mg. The pharmacokinetics were determined using a population-based approach combining full profiles (42 patients) and sparse data (218 patients). Parasite clearance time and 28 day cure rate were correlated with the derived pharmacokinetic parameters.
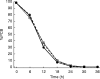
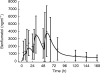
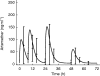
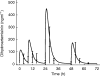
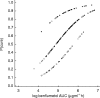
Results: The median absorption half-life of benflumetol was 5.3 h, with a tmax of 10 h and terminal elimination half-life of 4.5 days. For artemether (and its metabolite, dihydroartemisinin), the corresponding values were 1.9 (1.9) h, 1.8 (1.2) h, and 0.84 (0.43) h. The variability in bioavailability of artemether and dihydroartemisinin was large both between doses and between patients, but was less pronounced for benflumetol. Compared with the first dose, benflumetol bioavailability was estimated to increase three-fold by the third and fourth doses. Higher artemether or dihydroartemisinin AUC was found to decrease parasite clearance time. Higher benflumetol AUC was found to significantly increase the chance of cure.
Conclusions: Using a population-based approach it was confirmed that the pharmacokinetic and pharmacodynamic properties of benflumetol and artemether differ markedly. Benflumetol AUC is associated with cure and the effect of benflumetol when coadministered with artemether is to prevent recrudescence. The mode of action of benflumetol is consistent with its longer elimination half-life. A short course of low-dose artemether, which is rapidly absorbed and has a short elimination half-life, produced effective parasite clearance. The complementary pharmacokinetic and pharmacodynamic properties of benflumetol and artemether was the main rationale for developing a fixed-dose combination. While the 4 x 4 dose regimen is very effective in most endemic areas, the poorer absorption (2.5 fold lower than in China) and the more resistant parasites in Thailand require higher doses of this drug.
Figures
References
-
- Looareesuwan S, Harinasuta T, Chongsuphajaisiddhi T. Drug resistant malaria with special reference to Thailand. Southeast Asia J Trop Med Public Health. 1992;23:621–634. - PubMed
-
- Looareesuwan S, Vivaran C, Vanijanonta P, Wilairatana P, Pitisuttithum P, Andrial M. Comparative clinical trial of artesunate followed by mefloquine in the treatment of uncomplicated falciparum malaria: two- and three-day regimens. Am J Trop Med Hyg. 1996;54:210–213. - PubMed
-
- Mull R, Jiao Xeu-Qing. The comparative efficacy and tolerability of Coartem (CGP 56697) and benflumetol in the treatment of patients with plasmodium falciparum infection in China. 1998 (in preparation)
-
- de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. - PubMed
-
- Bunnag D, Viravan C, Looareesuwan S, Karbwang J, Harinasuta T. Clinical trial of artesunate and artemether on multidrug resistant P. falciparum malaria in Thailand. Southeast Asian J Trop Med Public Health. 1991;22:380–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

