Comparison of surface proteins of Anaplasma marginale grown in tick cell culture, tick salivary glands, and cattle
- PMID: 9864202
- PMCID: PMC96283
- DOI: 10.1128/IAI.67.1.102-107.1999
Comparison of surface proteins of Anaplasma marginale grown in tick cell culture, tick salivary glands, and cattle
Abstract
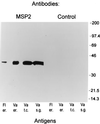
Anaplasma marginale, a tick-borne rickettsial pathogen of cattle, infects bovine erythrocytes, resulting in mild to severe hemolytic disease that causes economic losses in domestic livestock worldwide. Recently, the Virginia isolate of A. marginale was propagated in a continuous tick cell line, IDE8, derived from embryonic Ixodes scapularis. Development of A. marginale in cell culture was morphologically similar to that described previously in ticks. In order to evaluate the potential of the cell culture-derived organisms for use in future research or as an antigen for serologic tests and vaccines, the extent of structural conservation of the major surface proteins (MSPs) between the cell culture-derived A. marginale and the bovine erythrocytic stage, currently the source of A. marginale antigen, was determined. Structural conservation on the tick salivary-gland stage was also examined. Monoclonal and monospecific antisera against MSPs 1 through 5, initially characterized against erythrocyte stages, also reacted with A. marginale from cell culture and tick salivary glands. MSP1a among geographic A. marginale isolates is variable in size because of different numbers of a tandemly repeated 28- or 29-amino-acid peptide. The cell culture-derived A. marginale maintained the same-size MSP1a as that found on the Virginia isolate of A. marginale in bovine erythrocytes and tick salivary glands. Although differences were observed in the polymorphic MSP2 antigen between culture and salivary-gland stages, MSP2 did not appear to vary, by two-dimensional gel electrophoresis, during continuous passage in culture. These data show that MSPs of erythrocyte-stage A. marginale are present on culture stages and may be structurally conserved during continuous culture. The presence of all current candidate diagnostic and vaccine antigens suggests that in vitro cultures are a valuable source of rickettsiae for basic research and for the development of improved diagnostic reagents and vaccines against anaplasmosis.
Figures




References
-
- Alderink F J, Dietrich R A. Proceedings of the 86th Annual Meeting of the U.S. Animal Health Association; 1982. Economic and epidemiological implications of anaplasmosis in Texas cattle herds; pp. 65–75.
-
- Barbet A F. Recent developments in the molecular biology of anaplasmosis. Vet Parasitol. 1995;57:43–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

