Response to reactive nitrogen intermediates in Mycobacterium tuberculosis: induction of the 16-kilodalton alpha-crystallin homolog by exposure to nitric oxide donors
- PMID: 9864257
- PMCID: PMC96338
- DOI: 10.1128/IAI.67.1.460-465.1999
Response to reactive nitrogen intermediates in Mycobacterium tuberculosis: induction of the 16-kilodalton alpha-crystallin homolog by exposure to nitric oxide donors
Abstract
In contrast to the apparent paucity of Mycobacterium tuberculosis response to reactive oxygen intermediates, this organism has evolved a specific response to nitric oxide challenge. Exposure of M. tuberculosis to NO donors induces the synthesis of a set of polypeptides that have been collectively termed Nox. In this work, the most prominent Nox polypeptide, Nox16, was identified by immunoblotting and by N-terminal sequencing as the alpha-crystallin-related, 16-kDa small heat shock protein, sHsp16. A panel of chemically diverse donors of nitric oxide, with the exception of nitroprusside, induced sHsp16 (Nox16). Nitroprusside, a coordination complex of Fe2+ with a nitrosonium (NO+) ion, induced a 19-kDa polypeptide (Nox19) homologous to the nonheme bacterial ferritins. We conclude that the NO response in M. tuberculosis is dominated by increased synthesis of the alpha-crystallin homolog sHsp16, previously implicated in stationary-phase processes and found in this study to be a major M. tuberculosis protein induced upon exposure to reactive nitrogen intermediates.
Figures


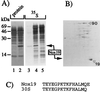
References
-
- Altuvia S, Almirom M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and ςS in stationary phase. Mol Microbiol. 1994;13:265–272. - PubMed
-
- Ang S C, Moscovic E A. Cross-reactive and species specific Mycobacterium tuberculosis antigens in the immunoprofile of Schaumann bodies: a major clue to the etiology of sarcoidosis. Histol Histopathol. 1996;11:125–134. - PubMed
-
- Bermudez E L, Young L S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–3013. - PubMed
-
- Bozzi M, Mignogna G, Stefanini S, Barr D, Longhi C, Valenti P, Chiancone E. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J Biol Chem. 1997;272:3259–3265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

