Adaptation of Mycobacterium smegmatis to stationary phase
- PMID: 9864340
- PMCID: PMC103559
- DOI: 10.1128/JB.181.1.270-283.1999
Adaptation of Mycobacterium smegmatis to stationary phase
Abstract
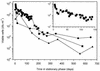
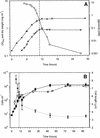
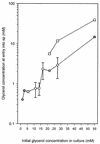
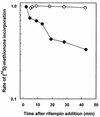
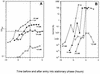
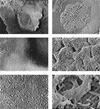
Mycobacterium tuberculosis can persist for many years within host lung tissue without causing clinical disease. Little is known about the state in which the bacilli survive, although it is frequently referred to as dormancy. Some evidence suggests that cells survive in nutrient-deprived stationary phase. Therefore, we are studying stationary-phase survival of Mycobacterium smegmatis as a model for mycobacterial persistence. M. smegmatis cultures could survive 650 days of either carbon, nitrogen, or phosphorus starvation. In carbon-limited medium, cells entered stationary phase before the carbon source (glycerol) had been completely depleted and glycerol uptake from the medium continued during the early stages of stationary phase. These results suggest that the cells are able to sense when the glycerol is approaching limiting concentrations and initiate a shutdown into stationary phase, which involves the uptake of the remaining glycerol from the medium. During early stationary phase, cells underwent reductive cell division and became more resistant to osmotic and acid stress and pool mRNA stabilized. Stationary-phase cells were also more resistant to oxidative stress, but this resistance was induced during late exponential phase in a cell-density-dependent manner. Upon recovery in fresh medium, stationary-phase cultures showed an immediate increase in protein synthesis irrespective of culture age. Colony morphology variants accumulated in stationary-phase cultures. A flat colony variant was seen in 75% of all long-term-stationary-phase cultures and frequently took over the whole population. Cryo scanning electron microscopy showed that the colony organization was different in flat colony strains, flat colonies appearing less well organized than wild-type colonies. Competition experiments with an exponential-phase-adapted wild-type strain showed that the flat strain had a competitive advantage in stationary phase, as well a providing evidence that growth and cell division occur in stationary-phase cultures of M. smegmatis. These results argue against stationary-phase M. smegmatis cultures entering a quiescent state akin to dormancy but support the idea that they are a dynamic population of cells.
Figures



References
-
- Albertson N H, Nystrom T, Kjelleberg S. Functional mRNA half-lives in the marine Vibrio sp. S14 during starvation and recovery. J Gen Microbiol. 1990;136:2195–2199.
-
- Albertson N H, Nystrom T, Kjelleberg S. Macromolecular synthesis during recovery of the marine Vibrio sp. S14 from starvation. J Gen Microbiol. 1990;136:2201–2207.
-
- Barer M R. Viable but non-culturable and dormant bacteria: time to resolve an oxymoron and a misnomer? J Med Microbiol. 1997;46:629–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

