Activation of alphaVbeta3 on vascular cells controls recognition of prothrombin
- PMID: 9864377
- PMCID: PMC2175236
- DOI: 10.1083/jcb.143.7.2081
Activation of alphaVbeta3 on vascular cells controls recognition of prothrombin
Abstract
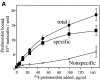
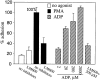
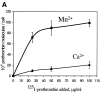
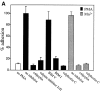
Regulation of vascular homeostasis depends upon collaboration between cells of the vessel wall and blood coagulation system. A direct interaction between integrin alphaVbeta3 on endothelial cells and smooth muscle cells and prothrombin, the pivotal proenzyme of the blood coagulation system, is demonstrated and activation of the integrin is required for receptor engagement. Evidence that prothrombin is a ligand for alphaVbeta3 on these cells include: (a) prothrombin binds to purified alphaVbeta3 via a RGD recognition specificity; (b) prothrombin supports alphaVbeta3-mediated adhesion of stimulated endothelial cells and smooth muscle cells; and (c) endothelial cells, either in suspension and in a monolayer, recognize soluble prothrombin via alphaVbeta3. alphaVbeta3-mediated cell adhesion to prothrombin, but not to fibrinogen, required activation of the receptor. Thus, the functionality of the alphaVbeta3 receptor is ligand defined, and prothrombin and fibrinogen represent activation- dependent and activation-independent ligands. Activation of alphaVbeta3 could be induced not only by model agonists, PMA and Mn2+, but also by a physiologically relevant agonist, ADP. Inhibition of protein kinase C and calpain prevented activation of alphaVbeta3 on vascular cells, suggesting that these molecules are involved in the inside-out signaling events that activate the integrin. The capacity of alphaVbeta3 to interact with prothrombin may play a significant role in the maintenance of hemostasis; and, at a general level, ligand selection by alphaVbeta3 may be controlled by the activation state of this integrin.
Figures










Similar articles
-
Networking in the hemostatic system. Integrin alphaiibbeta3 binds prothrombin and influences its activation.J Biol Chem. 1997 Oct 24;272(43):27183-8. doi: 10.1074/jbc.272.43.27183. J Biol Chem. 1997. PMID: 9341161
-
Integrin alphavbeta6 mediates HT29-D4 cell adhesion to MMP-processed fibrinogen in the presence of Mn2+.Eur J Cell Biol. 2007 Mar;86(3):143-60. doi: 10.1016/j.ejcb.2006.12.002. Epub 2007 Feb 1. Eur J Cell Biol. 2007. PMID: 17275949
-
Ligation of CD31 (PECAM-1) on endothelial cells increases adhesive function of alphavbeta3 integrin and enhances beta1 integrin-mediated adhesion of eosinophils to endothelial cells.Blood. 1999 Aug 15;94(4):1319-29. Blood. 1999. PMID: 10438720
-
Integrins and signaling in osteoclast function.Matrix Biol. 2000 May;19(2):97-105. doi: 10.1016/s0945-053x(00)00051-2. Matrix Biol. 2000. PMID: 10842093 Review.
-
Fibrinogen signal transduction in the nervous system.J Thromb Haemost. 2009 Jul;7 Suppl 1(Suppl 1):151-4. doi: 10.1111/j.1538-7836.2009.03438.x. J Thromb Haemost. 2009. PMID: 19630789 Free PMC article. Review.
Cited by
-
Actual Role of Platelet Glycoprotein IIb/IIIa Receptor Inhibitors as Adjunctive Pharmacological Therapy to Primary Angioplasty in Acute Myocardial Infarction: In the Light of Recent Randomized Trials and Observational Studies with Bivalirudin.Open Cardiovasc Med J. 2010 Jun 17;4:135-45. doi: 10.2174/1874192401004010135. Open Cardiovasc Med J. 2010. PMID: 20700394 Free PMC article.
-
Early Bunyavirus-Host Cell Interactions.Viruses. 2016 May 24;8(5):143. doi: 10.3390/v8050143. Viruses. 2016. PMID: 27213430 Free PMC article. Review.
-
αVβ3 integrin regulates macrophage inflammatory responses via PI3 kinase/Akt-dependent NF-κB activation.J Cell Physiol. 2011 Feb;226(2):469-76. doi: 10.1002/jcp.22356. J Cell Physiol. 2011. PMID: 20672329 Free PMC article.
-
Oral glycoprotein IIb/IIIa antagonists in coronary artery disease.Curr Cardiol Rep. 2001 Jan;3(1):63-71. doi: 10.1007/s11886-001-0012-2. Curr Cardiol Rep. 2001. PMID: 11139801 Review.
-
A New Strategy for Deleting Animal drugs from Traditional Chinese Medicines based on Modified Yimusake Formula.Sci Rep. 2017 May 4;7(1):1504. doi: 10.1038/s41598-017-01613-7. Sci Rep. 2017. PMID: 28473709 Free PMC article.
References
-
- Bennett JS, Chan C, Vilaire G, Mousa SA, DeGrado WF. Agonist-activated αVβ3on platelets and lymphocytes binds to the matrix protein osteopontin. J Biol Chem. 1997;272:8137–8140. - PubMed

