Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus
- PMID: 9869593
- PMCID: PMC89048
- DOI: 10.1128/AAC.43.1.190
Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus
Abstract
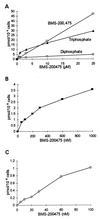
BMS-200475 was recently shown to have potent antiviral activity against hepatitis B virus (50% effective concentration = 3.7 nM; 50% cytotoxic concentration = 30 microM). In metabolic studies in both HepG2 and hepatitis B virus-transfected 2.2.15 human hepatoma cell lines, the metabolism was similar, the primary products being the di- and triphosphates. The accumulation of triphosphate was rapid and detectable down to a 5 nM concentration of added drug. When cells were labeled at 25 microM, the intracellular triphosphate concentration attained 30 pmol/10(6) cells ( approximately 30 microM). The intracellular half-life of the triphosphate was about 15 h. Compared with five other nucleoside analogs of medical interest (lamivudine, penciclovir, ganciclovir, acyclovir, and lobucavir), BMS-200475 was most efficiently phosphorylated to the triphosphate in HepG2 cells.
Figures


References
-
- Beasley R P, Hwang L Y. Overview on the epidemiology of hepatocellular carcinoma. In: Hollinger F B, Lemon S M, Margolis M, editors. Viral hepatitis and liver disease. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 532–535.
-
- Bisacchi G S, Chao S T, Bachard C, Daris J P, Innaimo S, Jacobs G A, Kocy O, Lapointe P, Martel A, Merchant Z, Slusarchyk W A, Sundeen J E, Young M G, Colonno R, Zahler R. BMS-200475, a novel carbocyclic 2′-deoxyguanosine analog with potent and selective anti-hepatitis B virus activity in vitro. Bioorg Med Chem Lett. 1997;7:127–132.
-
- Cammack N, Rouse P, Marr C L P, Reid P J, Boehme R E, Coates J A V, Penn C R, Cameron J M. Cellular metabolism of (−)enantiomeric 2′-deoxy-3′-thiacytidine. Biochem Pharmacol. 1992;43:2059–2064. - PubMed
-
- Chang C-N, Skalski V, Zhou J H, Cheng Y-C. Biochemical pharmacology of (+)- and (−)-2′,3′-dideoxy-3′-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992;267:22414–22420. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

