Molecular interactions between Vestigial and Scalloped promote wing formation in Drosophila
- PMID: 9869635
- PMCID: PMC317270
- DOI: 10.1101/gad.12.24.3815
Molecular interactions between Vestigial and Scalloped promote wing formation in Drosophila
Abstract
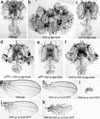
Scalloped (Sd) and Vestigial (Vg) are each needed for Drosophila wing development. We show that Sd is required for Vg function and that altering their relative cellular levels inhibits wing formation. In vitro, Vg binds directly to both Sd and its human homolog, Transcription Enhancer Factor-1. The interaction domains map to a small region of Vg that is essential for Vg-mediated gene activation and to the carboxy-terminal half of Sd. Our observations indicate that Vg and Sd function coordinately to control the expression of genes required for wing development, which implies that Vg is a tissue-specific transcriptional intermediary factor of Sd.
Figures



References
-
- Blair SS. A role for the segment polarity gene shaggy-zeste white 3 in the specification of regional identity in the developing wing of Drosphila. Dev Biol. 1994;162:229–244. - PubMed
-
- Blochlinger KR, Bodmer R, Jan LY, Jan YN. Patterns of expression of cut, a protein required for external sensory organ development in wild type and mutant Drosphila embryos. Genes & Dev. 1990;4:1322–1331. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Campbell SD, Inamdar M, Rodrigues V, Raghavan V, Palazzolo M, Chovnick A. The scalloped gene encodes a novel, evolutionarily conserved transcription factor required for sensory organ differentiation in Drosphila. Genes & Dev. 1992;6:367–379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
